Just Published in Angewandte Chemie, International Edition
27.01.2017Protonation versus Oxonium Salt Formation: Basicity and Stability Tuning of Cyanoborate Anions
Authors: Christoph Kerpen, Dr. Jan A. P. Sprenger, Lorena Herkert, Marius Schäfer, Lisa A. Bischoff, Pierre Zeides, Dr. Matthias Grüne, Dr. Rüdiger Bertermann, Franziska A. Brede, Prof. Dr. Klaus Müller-Buschbaum, Dr. Nikolai V. Ignat'ev, Prof. Dr. Maik Finze
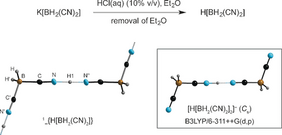
Abstract: Anhydrous H[BH2(CN)2] crystallizes from acidic aqueous solutions of the dicyanodihydridoborate anion. The formation of H[BH2(CN)2] is surprising as the protonation of nitriles requires strongly acidic and anhydrous conditions but it can be rationalized based on theoretical data. In contrast, [BX(CN)3]− (X=H, F) gives the expected oxonium salts (H3O)[BX(CN)3] while (H3O)[BF2(CN)2]/H[BF2(CN)2] is unstable. H[BH2(CN)2] forms chains via N−H⋅⋅⋅N bonds in the solid state and melts at 54 °C. Solutions of H[BH2(CN)2] in the room-temperature ionic liquid [EMIm][BH2(CN)2] contain the [(NC)H2BCN−H⋅⋅⋅NCBH2(CN)]−anion and are unusually stable, which enabled the study of selected spectroscopic and physical properties. [(NC)H2BCN−H⋅⋅⋅NCBH2(CN)]−slowly gives H2 and [(NC)H2BCN−BH(CN)2]−. The latter compound is a source of the free Lewis acid BH(CN)2, as shown by the generation of [BHF(CN)2]− and BH(CN)2⋅py.
Link: http://onlinelibrary.wiley.com/doi/10.1002/anie.201611901/full