Transition metal assisted activation of relatively inert and strong element element bonds
Familiar to anyone who has taken basic chemistry courses, small molecules like H2, N2, O2 H2O, NH3, and CH4 or simple organic and inorganic molecules provide the ultimate reservoir for the synthesis of more complex compounds. However, these small molecules are generally thermodynamically quite stable and their successful utilization often depends critically on surmounting often quite significant kinetic barriers. It has long been recognized that transition metal ions play an important role in providing low-barrier reaction pathways through binding these molecules, through activation of element element bonds in the resulting complexes and through the utilization of these activated molecules in reactions within the coordination sphere of the transition metal complexes. Currently we are mainly interested in the activation of very strong element element bonds such as the N-N bond of dinitrogen, or C-F, C-H and C-C bonds of organic molecules or Si-O, Si-F, or B-F bonds in inorganic compounds.
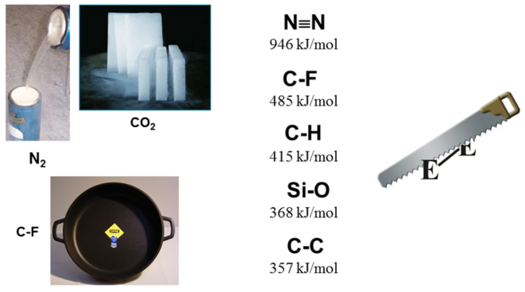
The carbon–fluorine bond, for example, is the strongest single bond in organic chemistry with exceptionally high bond dissociation energies having been reported. The high electronegativity of the fluorine substituent induces a significant ionic bond character resulting in a highly polarized, short C-F bond, which exhibits a low polarizability and a low-lying s* C-F antibonding orbital. Thus, fluorine substituents are only weak Lewis bases and fluoride itself is a poor leaving group. All these features add up to a high thermodynamic stability and often to a kinetic inertness of carbon–fluorine bonds (which, in fact, finds numerous applications, e.g. in Teflon® coated pans).
Fluorinated organic compounds increasingly gain importance in numerous areas of chemistry and everyday life. Building blocks which contain fluorinated entities are of high significance in areas such as materials science, catalysis, medicine, and biochemistry. Despite the numerous applications of fluorine-containing compounds, no known naturally occurring sources of aryl fluorides have been identified to date. As a result, aryl fluorides must be generated through chemical synthesis. Therefore, there is a growing demand for the development of unprecedented routes to access fluorinated molecules and building blocks. This can involve fluorination reactions as well as the introduction of fluorinated functionalities, or the selective derivatization of fluorinated compounds at a transition-metal center. For this purpose, the activation of C−F bonds has emerged to be an interesting methodology of fundamental interest, which enables to modify readily available by fluorinated building blocks.
We have shown, for example, that the reaction of [Ni2(iPr2Im)4(COD)] or [Ni(iPr2Im)2(η2-C2H4)] with different fluorinated arenes leads C-F bond activation of the fluorinated arene with a high chemo- and regioselectivity (T. Schaub, P. Fischer, A. Steffen, T. Braun, U. Radius, J. Am. Chem. Soc. 2008, 130, 9304–9317).
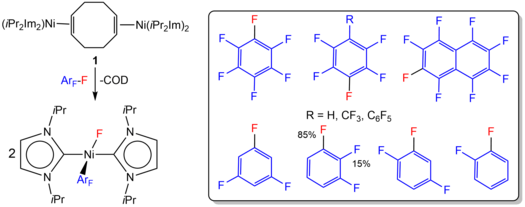
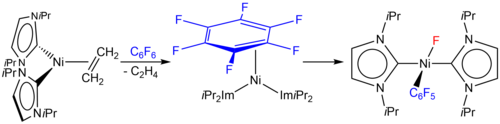
The detection of intermediates as well as kinetic studies give some insight into the mechanistic details for the activation of an aromatic carbon-fluorine bond at the {Ni(iPr2Im)2} complex fragment. The intermediates of the reaction of [Ni(iPr2Im)2(η2-C2H4)] with hexafluorobenzene and octafluoronaphthalene, [Ni(iPr2Im)2(η2-C6F6)] and [Ni(iPr2Im)2(η2-C10F8)], have been detected in solution. They convert into the C-F bond activation products. Complex [Ni(iPr2Im)2(η2-C10F8)] was structurally characterized by X-ray diffraction. The rates for the loss of this complex at different temperatures for the C-F activation of the coordinated naphthalene are first order and the estimated activation enthalpy DH¹ for this process was determined to be DH¹ = 116 ± 8 kJ mol-1 (DS¹ = 37 ± 25 J K-1 mol-1).
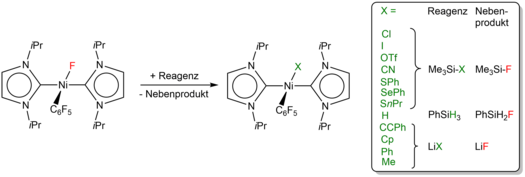
Furthermore, we have demonstrated that the NHC stabilized C-F-bond activation product [Ni(iPr2Im)2(F)(C6F5)] can easily be modified to give complexes [Ni(iPr2Im)2(X)(C6F5)] with halogenido, trifluoromethanesulfonato, cyanido, organyl, selenolato, thiolato, and hydrido ligands.