Reactivity of NHCs and related molecules with main group element hydrides and organyls
We recently extended our interest to investigations concerning the reactivity of NHCs with main group element hydride and alkyl/aryl compounds, respectively. These efforts are closely related to our activities in the area of NHC ring expansion (see below). In principle, the NHC can act here either as a Brönstedt or Lewis base leading to imidazolium salts or main group element NHC adducts, which may further react e.g. to give ring expansion products or other species.
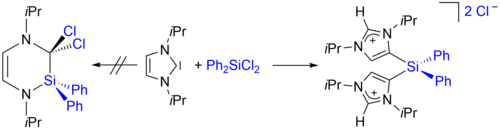
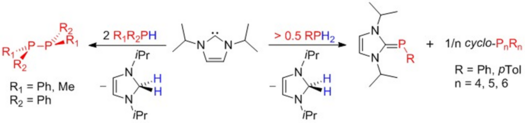
For example, we have reported recently (H. Schneider, D. Schmidt, U. Radius, Chem. Eur. J. 2015, 21, 2793-2797) that the reaction of iPr2Im (iPr2Im = 1,3-di-iso-propyl-imidazolin-2-ylidene) with diphenyldichlorosilane Ph2SiCl2 leads to the adduct (iPr2Im)SiCl2Ph2. Prolonged heating of isolated 1 in THF affords the backbone tethered di(imidazolium) salt [(aHiPr2Im)2SiPh2]2+2Cl- (“a” denotes “abnormal” coordination of the NHC). We have also demonstrate in a recent contribution that NHCs can be used in the dehydrogenative coupling of main group element hydrides. The dehydrogenative coupling of primary and secondary phosphines RPH2 and P2PH with iPr2Im leads to R2P–PR2 or to NHC phosphinidene adducts iPr2Im=PAr and cyclic oligophosphines P4Ar4, P5Ar5 and P6Ar6, respectively. The NHC acts in these reactions as phosphine activator and hydrogen acceptor.