Publications
| 65. | N. A. Jenek, A. Helbig, S. M. Boyt, M. Kaur, H. J. Sanderson, S. B. Reeksting, G. Kociok-Köhn, H. Helten,* U. Hintermair,* Understanding and tuning the electronic structure of pentalenides, Chem. Sci. 2024, accepted [DOI]. |
| 64. | M. Maier, F. Brosge, J. S. Schneider, J. Bachmann, S. Schmidt, C. Bolm,* H. Helten,* Sulfur-Based Building Blocks in BN- and BO-Doped Inorganic–Organic Hybrid Polymers, Macromolecules 2024, 57, 6370−6378 [DOI]. |
| 63. | F. Sturm, M, Bühler, C. Stapper, J. S. Schneider, H. Helten, I. Fischer,* M. I. Röhr,* Impact of isoelectronic substitution on the excited state processes in polycyclic aromatic hydrocarbons: a joint experimental and theoretical study of 4a,8a-azaboranaphthalene, Phys. Chem. Chem. Phys. 2024, 26, 7363−7370 [DOI] ("Hot Paper"). |
| 62. | M. Maier, A. Friedrich, J. S. Schneider, J. A. P. Sprenger, J. Klopf, L. Fritze, M. Finze, H. Helten,* Poly(iminoborane)s with Aromatic Side Groups: Insights into the Microstructure from Monodisperse Model Oligomers, ChemistryEurope 2024, 2, e202300085 [DOI]. Cover feature: [DOI] |
| 61. | M. Müller, H. Neitz, C. Höbartner,* H. Helten,* BN-Phenanthrene- and BN-Pyrene-Based Fluorescent Uridine Analogues, Org. Lett. 2024, 26, 1051–1055 [DOI]. |
| 60. | M. Maier, V. Zeh, N. Munker, J. Glock, K. Oberdorf, O. Ayhan, C. Lichtenberg, H. Helten,* 1,2,5-Azadiborolane as a Building Block for Inorganic–Organic Hybrid Polymers, Eur. J. Inorg. Chem. 2024, 27, e202300490 [DOI]. Front Cover: [DOI] |
59. | V. Zeh, J. S. Schneider, J. Bachmann, I. Krummenacher, H. Braunschweig, H. Helten,* Poly(ferrocenylene iminoborane): an inorganic–organic hybrid polymer comprising a backbone of moderately interacting ferrocenes, Chem. Commun. 2023, 59, 13723–13726 [DOI]. |
| 58. | M. Maier, J. Chorbacher, A. Hellinger, J. Klopf, J. Günther, H. Helten,* Poly(arylene iminoborane)s, Analogues of Poly(arylene vinylene) with a BN-Doped Backbone: A Comprehensive Study, Chem. Eur. J. 2023, 29, e202302767 [DOI] ("Hot Paper"). Cover feature: [DOI] |
| 57. | H. Helten,* R. Waterman,* In Honor of Prof. Dr. Dr. hc Rainer Streubel, Eur. J. Inorg. Chem. 2023, 26, e202300425 [DOI]. |
| 56. | J. S. Schneider, I. Krummenacher, H. Braunschweig, H. Helten,* Linear and macrocyclic oligo(p-phenylene iminoboranes) with ferrocenyl side groups – observation of selective, non-templated macrocyclization, Chem. Commun. 2023, 59, 8408–8411, [DOI] (special issue: Pioneering Investigators Collection 2023). |
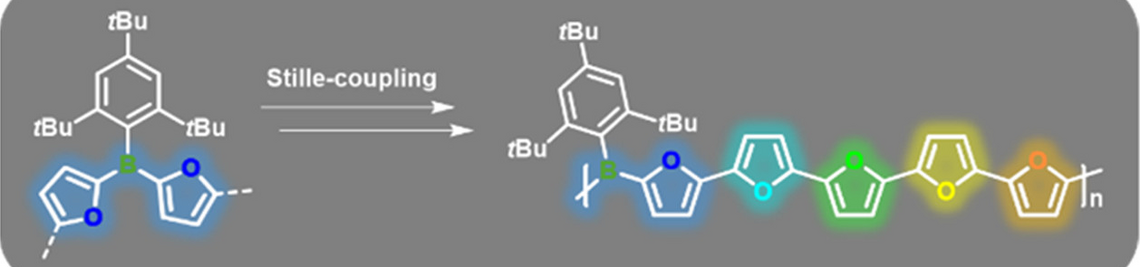
| 55. | J. Chorbacher, M. Maier, J. Klopf, M. Fest, H. Helten,* Poly(thiophene iminoborane): A Poly(thiophene vinylene) (PTV) Analogue with a Fully B=N‐Doped Backbone, Macromol. Rapid Commun. 2023, 44, 2300278, [DOI]. |
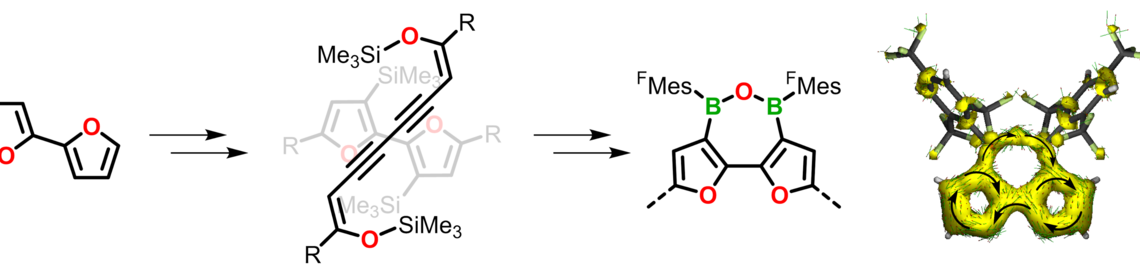
54. | J. Bachmann, A. Helbig, M. Crumbach, I. Krummenacher, H. Braunschweig, H. Helten,* Fusion of Aza- and Oxadiborepins with Furans via a Reversible Ring Opening Process Furnishes Versatile Building Blocks for Extended π-Conjugated Materials, Chem. Eur. J. 2022, 28, e202202455 [DOI]. |
| 53. | S. Hagspiel, F. Fantuzzi, M. Arrowsmith, A. Gärtner, M. Fest, J. Weiser, B. Engels, H. Helten, H. Braunschweig,* Modulation of the Naked-eye and Fluorescence Colour of a Protonated Boron-doped Thiazolothiazole by Anion-dependent Hydrogen Bonding, Chem. Eur. J. 2022, 28, e202201398 [DOI]. |
| 52. | M. Maier, J. Klopf, C. Glasmacher, F. Fantuzzi, J. Bachmann, O. Ayhan, A. Koner, B. Engels,* H. Helten,* Electrophilic activation of difunctional aminoboranes: B–N coupling versus intramolecular Cl/Me exchange, Chem. Commun. 2022, 58, 4464–4467 [DOI] (themed collection: Boron Chemistry in the 21st Century: From Synthetic Curiosities to Functional Molecules). |
| 51. | S. Fuchs, A. Jayaraman, I. Krummenacher, L. Haley, M. Baštovanović, M. Fest, K. Radacki, H. Helten, H. Braunschweig,* Diboramacrocycles: reversible borole dimerisation—dissociation systems, Chem. Sci. 2022, 13, 2932–2938 [DOI]. |
| 50. | H. Helten,* "Organoboron and Related Group 13 Polymers", in Comprehensive Organometallic Chemistry IV (Eds.: D. O’Hare, K. Meyer, G. Parkin), Elsevier, New York, 2022, pp. 71–134 [DOI]. |
| 49. | F. Schorr, N. Schopper, N. Riensch, F. Fantuzzi, M. Neder, R. D. Dewhurst, T. Thiess, T. Brückner, K. Hammond, H. Helten, M. Finze, H. Braunschweig,* Controlled Synthesis of Oligomers Containing Main-Chain B(sp2)-B(sp2) Bonds, Chem. Eur. J. 2021, 27, 16043–16048 [DOI]. |
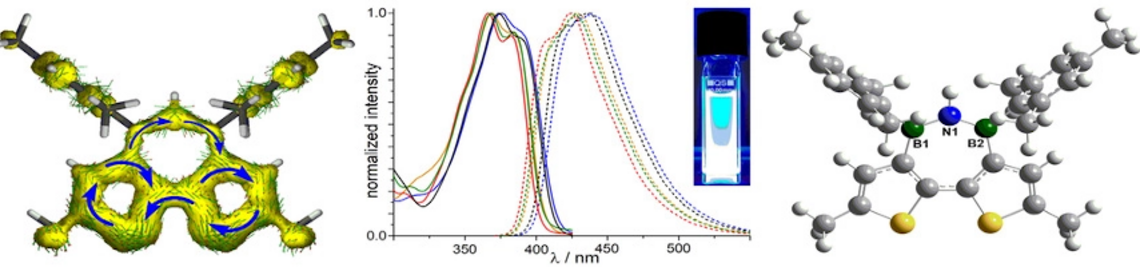
| 48. | L. Fritze, M. Fest, A. Helbig, T. Bischof, I. Krummenacher, H. Braunschweig, M. Finze, H. Helten,* Boron-Doped α‑Oligo- and Polyfurans: Highly Luminescent Hybrid Materials, Color-Tunable through the Doping Density, Macromolecules 2021, 54, 7653–7665 [DOI]. |
| 47. | L. Hahn, L. Keßler, L. Polzin, L. Fritze, S. Forster, H. Helten, R. Luxenhofer,* ABA Type Amphiphiles with Poly(2-benzhydryl-2-oxazine) Moieties: Synthesis, Characterization and Inverse Thermogelation, Macromol. Chem. Phys. 2021, 2100114 [DOI]. |
| 46. | M. Crumbach, J. Bachmann, L. Fritze, A. Helbig, I. Krummenacher, H. Braunschweig, H. Helten,* Dithiophene-Fused Oxadiborepins and Azadiborepins: A New Class of Highly Fluorescent Heteroaromatics, Angew. Chem. Int. Ed. 2021, 60, 9290–9295 [DOI]; Angew. Chem. 2021, 133, 9376–9381 [DOI] ("Hot Paper"). Highlighted in: "BRB, Looking for Aromaticity", T. M. Swager, M. Chen, Synfacts 2021, 17, 0392 [DOI]. |
| 45. | M. Crumbach, O. Ayhan, L. Fritze, J. A. P. Sprenger, L. Zapf, M. Finze, H. Helten,* BNB-Doped phenalenyls – aromaticity switch upon one-electron reduction, Chem. Commun. 2021, 57, 2408–2411 [DOI]. |
| 44. | N. A. Riensch, L. Swoboda, A. Lik, I. Krummenacher, H. Braunschweig, H. Helten,* Conjugated Bis(triarylboranes) with Disconnected Conjugation, Z. Anorg. Allg. Chem. 2021, 647, 421–424 [DOI]. |
| 43. | N. A. Riensch, M. Fest, L. Fritze, A. Helbig, I. Krummenacher, H. Braunschweig, H. Helten,* Bifuran-bridged bisboranes: highly luminescent B-doped oligohetarenes, New J. Chem. 2021, 45, 14920–14924 [DOI]. |
| 42. | T. Beweries,* H. Helten,* Poly(aminoborane)s & Poly(iminoborane)s, Encyclopedia of Inorganic and Bioinorganic Chemistry (Ed.: R. A. Scott), Wiley, Chichester, 2020, [DOI]. |
| 41. | F. Brosge, T. Lorenz, H. Helten,* C. Bolm,* BN- and BO-Doped Inorganic—Organic Hybrid Polymers with Sulfoximine Core Units, Chem. Eur. J. 2019, 25, 12708–12711 [DOI] ("Hot Paper"). |
| 40. | H. Helten,* Doping the Backbone of π-Conjugated Polymers with Tricoordinate Boron: Synthesis Strategies and Emerging Applications, Chem. Asian J. 2019, 14, 919—935 [DOI] (Minireview, invited). |
| 39. | L. Fritze, N. A. Riensch, H. Helten,* Catalytic Si/B Exchange Condensation: A Green B—C Coupling Method That Provides Access to Monodisperse (Het)arylborane "Trimers", Synthesis 2019, 51, 399—406 [DOI] (Feature Article, invited). |
| 38. | A. Lik, S. Jenthra, L. Fritze, L. Müller, K.-N. Truong, H. Helten,* From Monodisperse Thienyl- and Furylborane Oligomers to Polymers — Modulating the Optical Properties Through the Hetarene Ratio, Chem. Eur. J. 2018, 24, 11961—11972 [DOI] ("Very Important Paper", special issue: 7th EuCheMS Chemistry Congress, Liverpool). |
37. | N. A. Riensch, L. Fritze, T. Schindler, M. Kremer, H. Helten,* Difuryl(supermesityl)borane: a versatile building block for extended π-conjugated materials, Dalton Trans. 2018, 47, 10399—10403 [DOI] (special issue: New Talent: Europe, invited). |
| 36. | O. Ayhan, N. A. Riensch, C. Glasmacher, H. Helten,* Cyclolinear Oligo- and Poly(iminoborane)s: The Missing Link in Inorganic Main Group Macromolecular Chemistry, Chem. Eur. J. 2018, 24, 5883—5894 [DOI] ("Hot Paper"). Highlighted in: ChemistryViews [DOI]. Highlighted in: "Trendbericht Anorganische Chemie: Hauptgruppenelemente", C. Hering-Junghans, C. Sindlinger, Nachr. Chem. 2019, 67, 46—64 [DOI]. |
| 35. | A. Lik, D. Kargin, S. Isenberg, Z. Kelemen,* R. Pietschnig,* H. Helten,* PBP bridged [3]ferrocenophane: a bisphosphanylborane with a redox trigger, Chem. Commun. 2018, 54, 2471—2474 [DOI]. Inside back cover: [DOI] |
| 34. | N. A. Riensch, A. Deniz, S. Kühl, L. Müller, A. Adams, A. Pich,* H. Helten,* Borazine-based inorganic—organic hybrid cyclomatrix microspheres by silicon/boron exchange precipitation polycondensation, Polym. Chem. 2017, 8, 5264—5268 [DOI]. Inside front cover: [DOI] |
| 33. | A. Lik, L. Fritze, L. Müller, H. Helten,* Catalytic B—C Coupling by Si/B Exchange: A Versatile Route to π-Conjugated Organoborane Molecules, Oligomers, and Polymers, J. Am. Chem. Soc. 2017, 139, 5692—5695 [DOI]. |
| 32. | T. Lorenz, M. Crumbach, T. Eckert, A. Lik, H. Helten,* Poly(p-phenylene iminoborane): A Boron—Nitrogen Analogue of Poly(p-phenylene vinylene), Angew. Chem. Int. Ed. 2017, 56, 2780—2784 [DOI]; Angew. Chem. 2017, 129, 2824—2828 [DOI] ("Hot Paper"). Highlighted in: "Iminoborane units add inorganic flavor to polymers", S. K. Ritter, Chem. Eng. News 2017, 95(8), 11 [DOI]. |
| 31. | H. Helten,* O. Ayhan, T. Lorenz, Bor und Stickstoff statt Kohlenstoff, Nachr. Chem. 2017, 65, 535—541 [DOI]. |
| 30. | H. Helten,* Conjugated Inorganic—Organic Hybrid Polymers, Encyclopedia of Inorganic and Bioinorganic Chemistry (Ed.: R. A. Scott), Wiley, Chichester, 2017, [DOI]. |
| 29. | O. Ayhan, T. Eckert, F. A. Plamper, H. Helten,* Poly(iminoborane)s: An Elusive Class of Main Group Polymers?, Angew. Chem. Int. Ed. 2016, 55, 13321—13325 [DOI]; Angew. Chem. 2016, 128, 13515—13519 [DOI]. |
| 28. | T. Lorenz, A. Lik, F. A. Plamper, H. Helten,* Dehydrocoupling and Silazane Cleavage Routes to Organic—Inorganic Hybrid Polymers with NBN Units in the Main Chain, Angew. Chem. Int. Ed. 2016, 55, 7236—7241 [DOI]; Angew. Chem. 2016, 128, 7352—7357 [DOI]. Highlighted in: "A New Route to Polymers with NBN Backbone", T. M. Swager, Q. Zhang, Synfacts 2016, 12, 0691 [DOI]. Highlighted in: "Trendbericht Makromolekulare Chemie 2016", M. Sommer, F. Wurm, Nachr. Chem. 2017, 65, 348—358 [DOI]. |
| 27. | H. Helten,* B=N Units as Part of Extended π-Conjugated Oligomers and Polymers, Chem. Eur. J. 2016, 22, 12972—12982 [DOI] (Concept Article, invited). |
| 26. | A. P. M. Robertson, E. M. Leitao, T. Jurca, M. F. Haddow, H. Helten,* G. C. Lloyd-Jones,* I. Manners,* Mechanisms of the Thermal and Catalytic Redistributions, Oligomerizations, and Polymerizations of Linear Diborazanes, J. Am. Chem. Soc. 2013, 135, 12670—12683 [DOI]. |
| 25. | H. Helten, B. Dutta, J. R. Vance, M. E. Sloan, M. F. Haddow, S. Sproules, D. Collison, G. R. Whittell, G. C. Lloyd-Jones, I. Manners, Paramagnetic Titanium(III) and Zirconium(III) Metallocene Complexes as Precatalysts for the Dehydrocoupling/Dehydrogenation of Amine—Boranes, Angew. Chem. Int. Ed. 2013, 52, 437—440; Angew. Chem. 2013, 125, 455—458 ("Very Important Paper"). |
| 24. | E. M. Leitao, N. E. Stubbs, A. P. M. Robertson, H. Helten, R. J. Cox, G. C. Lloyd-Jones, I. Manners, Mechanism of Metal-Free Hydrogen Transfer between Amine—Boranes and Aminoboranes, J. Am. Chem. Soc. 2012, 134, 16805—16816. |
| 23. | V. Blackstone, S. Pfirrmann, H. Helten, A. Staubitz, A. Presa Soto, G. R. Whittell, I. Manners, A Cooperative Role for the Counteranion in the PCl5-Initiated Living, Cationic Chain Growth Polycondensation of the Phosphoranimine Cl3P=NSiMe3, J. Am. Chem. Soc. 2012, 134, 15293—15296. |
| 22. | H. Helten, A. P. M. Robertson, A. Staubitz, J. R. Vance, M. F. Haddow, I. Manners, “Spontaneous” Ambient Temperature Dehydrocoupling of Aromatic Amine—Boranes, Chem. Eur. J. 2012, 18, 4665—4680. |
| 21. | J. Marinas Pérez, H. Helten, G. Schnakenburg, R. Streubel, A Novel Approach to 1,3,4-Dioxaphospholane Complexes: Acid-Induced Ring Expansion of an Oxaphosphirane Complex. The Problem of Construction and Deconstruction of O,P-Heterocycles, Chem. Asian J. 2011, 6, 1539—1545. |
| 20. | H. Helten, G. Schnakenburg, J. Daniels, A. J. Arduengo, III, R. Streubel, When Sterics Overcome Electronics: An Unusual Haptotropic P→N Pentacarbonyltungsten Shift, Organometallics 2011, 30, 84—91. |
| 19. | H. Helten, G. Schnakenburg, R. Streubel, Click Reactions of 2H-Azaphosphirene Chromium and Molybdenum Complexes and a Surprisingly Facile Access to a 2H-1,4,2-Diazaphosphole Derivative, Polyhedron 2011, 30, 1799—1805. |
| 18. | J. Marinas Pérez, H. Helten, B. Donnadieu, C. A. Reed, R. Streubel, Protonation-Induced Rearrangement of an Oxaphosphirane Complex, Angew. Chem. Int. Ed. 2010, 49, 2615—2618; Angew. Chem. 2010, 122, 2670—2674. |
| 17. | J. Marinas Pérez, C. Albrecht, H. Helten, G. Schnakenburg, R. Streubel, Competing ring cleavage of transient O-protonated oxaphosphirane complexes: 1,3-oxaphospholane and η2-Wittig ylide complex formation, Chem. Commun. 2010, 46, 7244—7246. |
| 16. | R. Streubel, J. Marinas Pérez, H. Helten, J. Daniels, M. Nieger, Developing click reactions of a 3-ferrocenyl-2H-azaphosphirene complex, Dalton Trans. 2010, 39, 11445—11450. |
| 15. | S. Fankel, H. Helten, G. von Frantzius, G. Schnakenburg, J. Daniels, V. Chu, C. Müller, R. Streubel, Novel access to azaphosphiridine complexes and first applications using Brønsted acid-induced ring expansion reactions, Dalton Trans. 2010, 39, 3472—3481. |
| 14. | H. Helten, M. Beckmann, G. Schnakenburg, R. Streubel, Synthesis and Reactivity of an Unusual Ferrocenophane Bis(carbene complex), Eur. J. Inorg. Chem. 2010, 2337—2341. |
| 13. | H. Helten, J. Daniels, M. Nieger, R. Streubel, Extended π conjugation in 2H-1,4,2-diazaphosphole complexes, New J. Chem. 2010, 34, 1593—1602. |
| 12. | H. Helten, M. Engeser, D. Gudat, R. Schilling, G. Schnakenburg, M. Nieger, R. Streubel, Protonation of 2H-Azaphosphirene Complexes: P—N Bond Activation and Ring-Expansion Reactions, Chem. Eur. J. 2009, 15, 2602—2616. |
| 11. | H. Helten, J. Marinas Pérez, J. Daniels, R. Streubel, First Brønsted Acid-Induced Ring Expansion of an Oxaphosphirane Complex: A Combined Experimental and DFT Study, Organometallics 2009, 28, 1221—1226. |
| 10. | H. Helten, S. Fankel, O. Feier-Iova, M. Nieger, A. Espinosa Ferao, R. Streubel, Strong Evidence for an Unprecedented Borderline Case of Dissociation and Cycloaddition in Open-Shell 1,3-Dipole Chemistry: Transient Nitrilium Phosphane-Ylide Complex Radical Cations, Eur. J. Inorg. Chem. 2009, 3226—3237. |
| 9. | H. Helten, G. von Frantzius, G. Schnakenburg, J. Daniels, R. Streubel, How To Tune Acid-Induced Ring Enlargement Reactions — The Strange Case of 2H-Azaphosphirene Complexes and Its Surprising Dichotomy, Eur. J. Inorg. Chem. 2009, 2062—2065. |
| 8. | R. Streubel, M. Beckmann, C. Neumann, S. Fankel, H. Helten, O. Feier-Iova, P. G. Jones, M. Nieger, Synthesis, Structure, and Ring-Expansion Reactions of a 3-Ferrocenyl-Substituted 2H-Azaphosphirene Tungsten Complex, Eur. J. Inorg. Chem. 2009, 2090—2095. |
| 7. | H. Helten, C. Neumann, A. Espinosa, P. G. Jones, M. Nieger, R. Streubel, Evidence for Ligand-Centered Reactivity of a 17e Radical Cationic 2H-Azaphosphirene Complex, Eur. J. Inorg. Chem. 2007, 4669—4678. |
| 6. | R. Vicik, H. Helten, T. Schirmeister, B. Engels, Rational Design of Aziridine-Containing Cysteine Protease Inhibitors with Improved Potency: Studies on Inhibition Mechanism, ChemMedChem 2006, 1, 1021—1028. |
| 5. | H. Helten, T. Schirmeister, B. Engels, Theoretical Studies about the Influence of Different Ring Substituents on the Nucleophilic Ring Opening of Three-Membered Heterocycles and Possible Implications for the Mechanisms of Cysteine Protease Inhibitors, J. Org. Chem. 2005, 70, 233—237. |
| 4. | H. Helten, T. Schirmeister, B. Engels, Model Calculations about the Influence of Protic Environments on the Alkylation Step of Epoxide, Aziridine, and Thiirane Based Cysteine Protease Inhibitors, J. Phys. Chem. A 2004, 108, 7691—7701. |
| 3. | P. W. Musch, C. Remenyi, H. Helten, B. Engels, On the Regioselectivity of the Cyclization of Enyne-Ketenes: A Computational Investigation and Comparison with the Myers-Saito and Schmittel Reaction, J. Am. Chem. Soc. 2002, 124, 1823—1828. |
| 2. | B. Walfort, T. Auth, B. Degel, H. Helten, D. Stalke, Copper and Silver Triimidosulfites: S(NtBu)32--Bicapped M3-Triangles Connected via Lithium Halide Ladders or Fragments Thereof, Organometallics 2002, 21, 2208—2214. |
| 1. | M. Schmittel, J.-P. Steffen, M. Maywald, B. Engels, H. Helten, P. Musch, Ring size effects in the C2—C6 biradical cyclisation of enyne-allenes and the relevance for neocarzinostatin, J. Chem. Soc., Perkin Trans. 2 2001, 1331—1339. |