Mass Spectrometry
High-resolution mass spectrometry of bioconjugates
An important aspect of the research in the Schatzschneider group is directed at the synthesis of organometal peptide and protein bioconjugates using bioorthogonal "click" reactions. The method of choice to characterize such high molecular weight species is ESI mass spectrometry.
Thanks to a major instrument grant to the Marder group, we have direct access to a high-resolution ThermoFisher Exactive Plus Orbitrap mass spectrometer with ESI-HPLC, ESI, APCI, ASAP, and LIFDI ion sources. With a maximum resolution of R = 140.000 we routinely achieve an match between experimental and calculated masses below 1 mDa (0.001 Da), which allows baseline resolution even for +10 cations.
The instrument is custom-modified with a liquid injection field desorption/ionisation (LIFDI) ion source from Linden CMS, which allows extremely short switching times between measurement of FD and ESI mass spectra without the need to break the high vacuum.
For automated sample application and separation, the instrument can be fitted to an Ultimate 3000 HPLC system with autosampler and DAD detector, which allows correlation of the mass spectrometric signal with the UV/Vis signature of metal complexes and their bioconjugates.
Automated combinatorial synthesis
For a quick access to large chemical space, automated synthesis protocols based on the iClick reaction and using a re-purposed HPLC autosampler are developed in the group.
- T. Zach, F. Geyer, B. Kiendl, J. Mößeler, O. Nguyen, T. Schmidpeter, P. Schuster, U. Schatzschneider
Electrospray mass spectrometry to study combinatorial iClick reactions and multiplexed kinetics of [Ru(N3)(N^N)(terpy)]PF6 with alkynes of different steric and electronic demand
Inorg. Chem. 62, 2982-2993 (2023)

Long-term stability of metal complexes
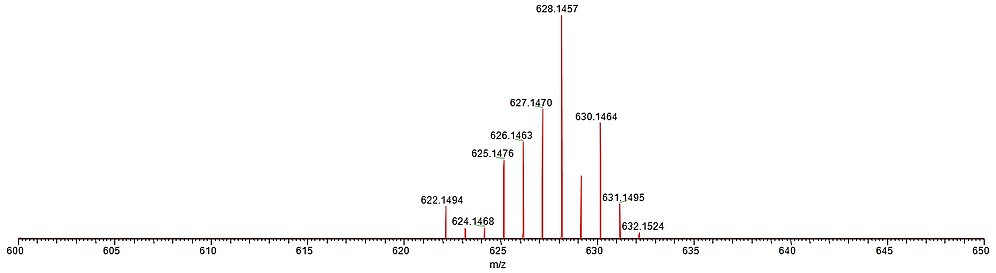
For biological and biomedical applications of metal complex bioconjugates, the long-term stability of the compounds under physiological conditions is a major issue. ESI mass spectrometry is a facile tool to study the speciation of charged metal complexes in aqueous solution under mild conditions.
The figure below shows the ESI mass spectrum of a Ru(II) arene complex after about 5 months of incubation in a methanol/water mixture in the dark at room temperature. Nicely visible is the extremely well-resolved isotope pattern mostly due to the Ru metal center, which is 100% identical to a spectrum obtained directly after dissolution of the sample.
Protein ESI mass spectrometry
Although the Exactive Plus mass spectrometer only has a measurement range of 50-6000 Da, higher molecular weight bio(macro)molecules can still be detected if they form multiply protonated species that "fold back" in the accessible mass range.
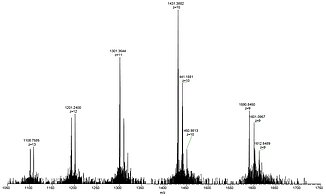
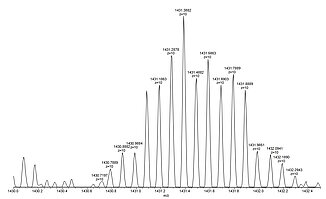
For example, lysozyme with a molecular weight of about 14.300 Da, in aqueous solution forms the [M+10H]10+ cation together with other multiply protonated species that can easily be detected by ESI mass spectrometry.
Shown below is a full view of the ESI mass spectrum of lysozyme in the range of 1050-1750 Da with the +9 to +13 multiply protonated species nicely visible (left), a close-up picture of the +10 cation showing the baseline resolution even for highly charged species (center), and the X-ray crystal structure of lysozyme taken from PDB 2VB1 (right).