Bioorthogonal "Click" Reactions
Metal-centered reactivity for the functionalization of bio(macro)molecules
Bio(macro)molecules such as peptides and proteins incorporate a large number of reactive functional groups. For the site-specific introduction of metal complex modifications under mild conditions, Click reactions are developed based on metal-centered reactivity for selective introduction of metal-based probe molecules.
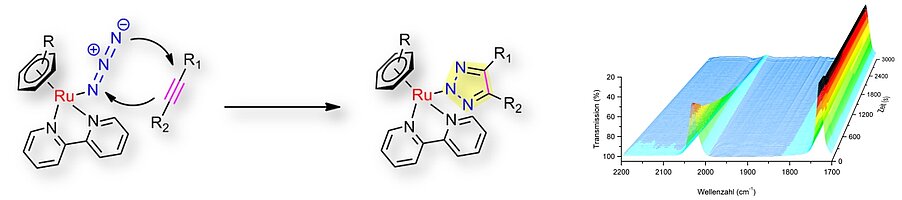
In particular, the iClick reaction of metal-coordinated azides groups with electron-poor alkynes holds great promise, since usually, it proceeds at room temperature without the need for catalyst addition:


- D. Moreth, G. Hörner, V. Müller, L. Geyer, U. Schatzschneider, An isostructural series of Ni(II), Pd(II), Pt(II), and Au(III) azido complexes with a N^C^N pincer ligand to elucidate trends in the iClick reaction kinetics and structural parameters of the triazolato products, Inorg. Chem. 62, 16000-16012 (2023)
- T. Zach, F. Geyer, B. Kiendl, J. Mößeler, O. Nguyen, T. Schmidpeter, P. Schuster, U. Schatzschneider, Electrospray mass spectrometry to study combinatorial iClick reactions and multiplexed kinetics of [Ru(N3)(N^N)(terpy)]PF6 with alkynes of different steric and electronic demand
Inorg. Chem. 62, 2982-2993 (2023) - K. Peng, D. Moreth, U. Schatzschneider, C^N^N coordination accelerates the iClick reaction of square-planar palladium(II) and platinum(II) azido complexes with electron-poor alkynes and enables cycloaddition with terminal alkynes
Organometallics 40, 2584-2593 (2021) - K. Peng, R. Einsele, P. Irmler, R. Winter, U. Schatzschneider, The iClick reaction of a BODIPY platinum(II) azido complex with electron-poor alkynes provides triazolate complexes with good 1O2 sensitization efficiency
Organometallics 39, 1423-1430 (2020) - K. Peng, V. Mawamba, E. Schulz, M. Löhr, C. Hagemann, U. Schatzschneider, iClick reactions of square-planar palladium(II) and platinum(II) azido complexes with electron-poor alkynes: Metal-dependent preference for N1 vs. N2 triazolate coordination and kinetic studies with 1H and 19F NMR spectroscopy, Inorg. Chem. 58, 11508-11521 (2019)
- P. Schmid, M. Maier, H. Pfeiffer, L. Henry, A. Belz, A. Friedrich, F. Schönfeld, K. Edkins, U. Schatzschneider, Catalyst-free room-temperature iClick reaction of molybdenum(II) and tungsten(II) azide complexes with electron-poor alkynes: Structural preferences and kinetic studies, Dalton Trans. 46, 13386-13396 (2017)
- L. Waag-Hiersch, J. Mößeler, U. Schatzschneider, Electronic influences on the stability and kinetics of Cp* rhodium(III) azide complexes in the iClick reaction with electron-poor alkynes, Eur. J. Inorg. Chem. 3024-3029 (2017)
- L. Henry, C. Schneider, B. Mützel, P.V. Simpson, C. Nagel, K. Fucke, U. Schatzschneider, Amino acid bioconjugation via iClick reaction of an oxanorbornadiene-masked alkyne with a MnI(bpy)(CO)3-coordinated azide, Chem. Commun. 50, 15692-15695 (2014)
- S. Pai, K. Radacki, U. Schatzschneider, Sonogashira, CuAAC, and oxime ligations for the synthesis of Mn(I) tricarbonyl PhotoCORM peptide conjugates, Eur. J. Inorg. Chem. 2886-2895 (2014)
- H. Pfeiffer, T. Sowik, U. Schatzschneider, Bioorthogonal oxime ligation of a Mo(CO)4(N-N) CO releasing molecule (CORM) to a TGF beta-binding peptide, J. Organomet. Chem. 734, 17-24 (2013)
- H. Pfeiffer, A. Rojas, J. Niesel, U. Schatzschneider, Sonogashira and "Click" reactions in the N-terminal and side chain functionalization of peptides with [Mn(CO)3(tpm)]+-based CO releasing molecules (tpm = tris(pyrazolyl)methane), Dalton Trans. 4292-4298 (2009)
Click reactions for the functionalization of hard nanomaterials
Click reactions can also be employed for the functionalization of hard nanomaterials. For example, the copper-catalyzed azide-alkyne (CuAAC) cycloaddition reaction between a surface-bound alkyl azide and an alkyne-functionalized manganese(I) tricarbonyl complex was utilized for the decoration of silica nanoparticles as well as nanodiamond with CO-releasing molecules (CORMs):
- G. Dördelmann, T. Meinhardt, T. Sowik, A. Krüger, U. Schatzschneider, CuAAC click functionalization of azide-modified nanodiamond with a photoactivatable CO releasing molecule (PhotoCORM) based on [Mn(CO)3(tpm)]+, Chem. Commun. 48, 11528-11530 (2012)
- G. Dördelmann, H. Pfeiffer, A. Birkner, U. Schatzschneider, Silicium dioxide nanoparticles as carriers for photoactivatable CO releasing molecules (PhotoCORMs), Inorg. Chem. 50, 4362-4367 (2011)