LAs (Lewis Acids) & WCAs (Weakly Coordinating Anions)
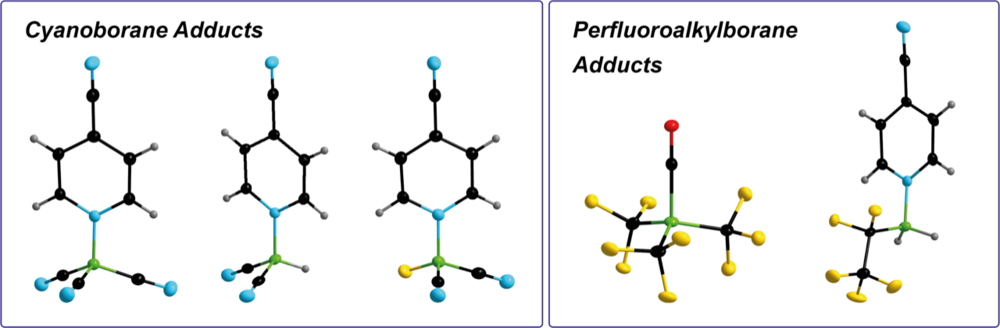
Cyanoborane & Perfluoroalkylborane Adducts
Cyanoboranes and perfluoroalkylboranes typically are strong Lewis acids and some of them are even Lewis superacids. Most cyano- and perfluoroalkylboranes are not stable as free, non-coordinated molecules as they either undergo polymerization via B–CN–B bond formation or they undergo inter- and intramolecular C–F activation. Thus, we are developing adducts of these Lewis acids that serve as synthons for the free Lewis acids.
Publications on this topic:
Boranes Paving the Way to Anionic Cyclic (Alkyl)(amino)carbenes (Ani-cAACs)
L. Zapf, S. Peters, M. Radius, M. Finze, Angew. Chem. Int. Ed. 2023, 62, e202300056.
The pentafluoroethyltrihydridoborate anion: from shock sensitive salts to stable room temperature ionic liquids
P. T. Hennig, J. A. P. Sprenger, L. N. Schneider, N. V. Ignat’ev, M. Finze, Chem. Commun. 2019, 55, 6110–6113.
Trifluoromethylboranes and -Borates: New Synthetic Strategies and Applications
M. Finze, E. Bernhardt, H. Willner, Angew. Chem. Int. Ed. 2007, 46, 9180–9196.
Cyanoborates
N. V. Ignat’ev, M. Finze, Eur. J. Inorg. Chem. 2019, 3539–3560.
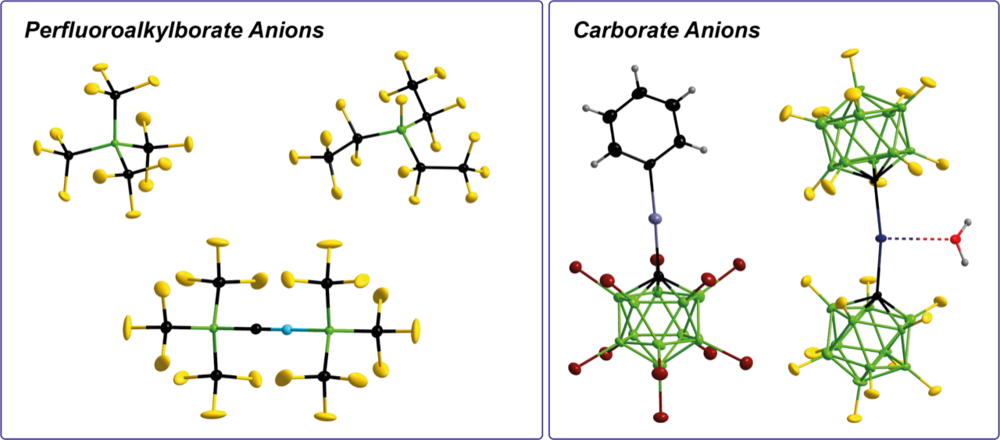
Boron-Based Weakly Coordinating Anions
Different types of boron-based weakly coordinating anions (WCAs) are in the focus of our current studies. For example, this includes perfluoroalkylborate anions and carba-closo-dodecaborate (carborate) anions. These WCAs are used for the stabilization of reactive main group element and transition metal cations, as building block for ionic liquids (ILs), or for electrolyte applications.
Publications on this topic:
Salts of the Lewis-Acidic Dianion [Hg(closo-1-CB11F11)2]2– Coordination of Acetonitrile and Water
A. Himmelspach, M. Zähres, M. Finze, Inorg. Chem. 2011, 50, 3186–3188.
Mercury(II) Complexes of the Carba-closo-dodecaboranyl Ligands [closo-1-CB11X11]2− (X = H, F, Cl, Br, I)
A. Himmelspach, J. A. P. Sprenger, J. Warneke, M. Zähres, M. Finze, Organometallics 2012, 31, 1566–1577.
Mechanistic Study on the Fluorination of K[B(CN)4] with ClF Enabling the High Yield and Large Scale Synthesis of K[B(CF3)4] and K[(CF3)3BCN]
E. Bernhardt, M. Finze, H. Willner, Inorg. Chem. 2011, 50, 10268–10273.
Trifluoromethylboranes and -Borates: New Synthetic Strategies and Applications
M. Finze, E. Bernhardt, H. Willner, Angew. Chem. Int. Ed. 2007, 46, 9180–9196.
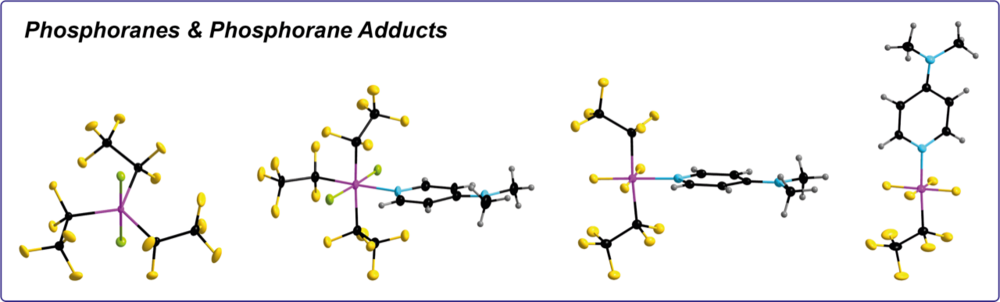
Perfluoroalkylphosphoranes
Tris(perfluoroalkyl)phosphoranes are synthesized via electrochemical fluorination (ECF). The follow-up chemistry of these phosphoranes, especially of (C2F5)3PF2, is investigated. It provides access to other phosphoranes, e.g. (C2F5)2PCl3, (C2F5)2PF3, and (C2F5)PF4, with tunable electronic and steric properties. We use these phosphoranes as precursors for weakly coordinating perfluoroalkylphosphate anions (ionic liquids, electrolytes, stabilization of cationic metal complexes), as Lewis acid organocatalysts, and for ligand design (NHCs and cAACs).
Publications on this topic:
An easy-to-perform evaluation of steric properties of Lewis acids
L. Zapf, M. Riethmann, S. A. Föhrenbacher, M. Finze, U. Radius, Chem. Sci. 2023, 14, DOI: 10.1039/D3SC00037K
Tris(pentafluoroethyl)difluorophosphorane and N-Heterocyclic Carbenes: Adduct Formation and Frustrated Lewis Pair Reactivity
S. A. Föhrenbacher, V. Zeh, M. J. Krahfuss, N. V. Ignat'ev, M. Finze, U. Radius, Eur. J. Inorg. Chem. 2021, 1941–1960.
Tris(pentafluoroethyl)difluorophosphorane: A Versatile Fluoride Acceptor for Transition Metal Chemistry
S. A. Föhrenbacher, M. J. Krahfuß, L. Zapf, A. Friedrich, N. V. Ignat’ev, M. Finze, M. Radius, Chem. Eur. J. 2021, 27, 3504–3516.
New Hydrophobic Ionic Liquids with Perfluoroalkylphosphate and Cyanofluoroborate Anions
N. V. Ignat’ev, M. Finze, J. A. P. Sprenger, C. Kerpen, E. Bernhardt, H. Willner, J. Fluorine Chem. 2015, 177, 46–54.
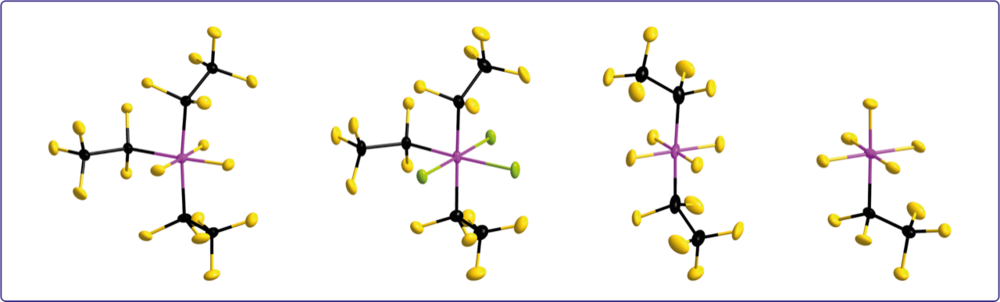
Perfluoroalkylphosphate Anions
Perfluoroalkylphosphate anions such as the [(C2F5)3PF3]– anion (FAP3) are versatile weakly coordinating anions (WCAs) with tunable properties, e.g. solubility. Since the conjugated free Lewis acids, perfluoroalkylphosphoranes, are readily accessible, storable, and easy-to-handle, perfluoroalkylphosphate anions are of interest for many different applications. We employ the free Lewis acids for the activation of metal fluorido complexes to give cationic complexes stabilized by perfluoroalkylphosphate anions.
Publications on this topic:
Tris(pentafluoroethyl)difluorophosphorane: A Versatile Fluoride Acceptor for Transition Metal Chemistry
S. A. Föhrenbacher, M. J. Krahfuß, L. Zapf, A. Friedrich, N. V. Ignat’ev, M. Finze, M. Radius, Chem. Eur. J. 2021, 27, 3504–3516.
New Hydrophobic Ionic Liquids with Perfluoroalkylphosphate and Cyanofluoroborate Anions
N. V. Ignat’ev, M. Finze, J. A. P. Sprenger, C. Kerpen, E. Bernhardt, H. Willner, J. Fluorine Chem. 2015, 177, 46–54.