Just Published in Zeitschrift für anorganische und allgemeine Chemie
04.10.2016To Rearrange or not to Rearrange: Reactivity of NHCs towards Chloro- and Hydrostannanes R2SnCl2 (R = Me, Ph) and Ph3SnH
Authors: Heidi Schneider, Mirjam J. Krahfuß, Udo Radius
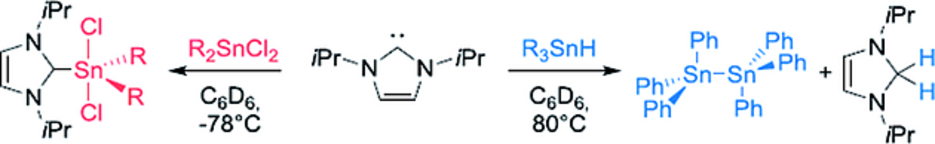
Abstract: The reaction of 1,3-diisopropylimidazolin-2-ylidene (iPr2Im) with diphenyldichlorostannane and dimethyldichlorostannane, respectively, leads to the formation of the adducts (iPr2Im)·SnPh2Cl2 (1) and (iPr2Im)·SnMe2Cl2 (2). These compounds are stable in solution to temperatures up to 80 °C for several days and rearrangement to backbone-tethered bis(imidazolium) salts or ring expansion reaction to six membered heterocyclic rings was not observed. The reaction ofiPr2Im with triphenylstannane Ph3SnH leads to reductive dehydrocoupling of the stannane to yield distannane Sn2Ph6 andiPr2ImH2. Thus, the reactivity of these tin compounds is completely different compared to those of the lighter congener silicon, for which rearrangement (chlorides) and NHC ring expansion (hydrides) was reported earlier.
Link: http://onlinelibrary.wiley.com/doi/10.1002/zaac.201600271/abstract