Just Published in Zeitschrift für anorganische und allgemeine Chemie
31.10.2016Unexpected Dimeric Spiro-Borate Complexes from Lewis-Acid Induced Transformation of Oxalatoborates
Authors: Sven H. Zottnick, Jens R. Sorg, Jan A. P. Sprenger, Maik Finze, Klaus Müller-Buschbaum
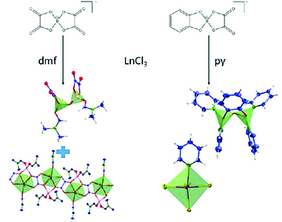
Abstract: Oxalatoborates were transformed into dimeric spiro-borate complexes by abstraction of oxalate ligands by Lewis acidic lanthanide trichlorides. Oxalatoborates with different anions and cations were investigated containing either bis-oxalatoborate or catecholato-oxalatoborate anions and lithium as well as EMIM (1-ethyl-3-methyl-imidazolium) and BMIM cations (1-n-butyl-3-methyl-imidazolium) from the respective ionic liquids (ILs). Depending on the amount of oxalate groups and the different cations, the oxalate groups are partly or fully abstracted and transferred to the lanthanides. The reaction of anhydrous PrCl3 with Li[B(C6H4O2)(C2O4)] (lithium catecholato-oxalatoborate = Li[Catbox]) in pyridine (py) led to a conversion of the borate anion to the complex [B2O(C6H4O2)(py)4][PrCl5(py)] (1). The use of the ILs [EMIM][Catbox] and [BMIM][Catbox] indicates complete transfer of the oxalate groups to the lanthanide ions in reactions with lanthanide chlorides and nitrates yielding the well-known lanthanide oxalates Ln2(C2O4)3·10H2O (Ln = La, Eu). In contrast, the reaction of anhydrous YCl3 with Li[B(C2O4)2] (lithium bis-oxalatoborate = Li[BOB]) in N,N-dimethylformamide (dmf) results in the formation of [B2O(C2O4)2(dmf)2] (2). Transfer of one oxalate group per boron onto the yttrium ion is revealed by the formation of the one-dimensional coordination polymer ∞1[LiYCl2(C2O4)(dmf)3] (3) as additional product. The products were characterized by single-crystal X-ray diffraction as well as powder X-ray diffraction, IR spectroscopy and elemental analysis.
Link: http://onlinelibrary.wiley.com/doi/10.1002/zaac.201600319/abstract