Just Published in Physical Chemistry Chemical Physics
19.12.2018Properties of perhalogenated {closo-B10} and {closo-B11} multiply charged anions and a critical comparison with {closo-B12} in the gas and the condensed phase
Authors: Jonas Warneke,* Szymon Z. Konieczk, Gao-Lei Hou, Edoardo Aprà, Christoph Kerpen, Fabian Keppner, Thomas C. Schäfer, Michael Deckert, Zheng Yang, Eric J. Bylaska, Grant E. Johnson, Julia Laskin, Sotiris S. Xantheas, Xue-Bin Wang and Maik Finze*
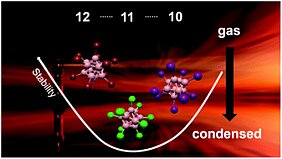
Abstract: closo-Borate anions [closo-BnXn]2− are part of the most famous textbook examples of polyhedral compounds. Substantial differences in their reactivity and interactions with other compounds depending on the substituent X and cluster size n have been recognized, which favor specific closo-borates for different applications in cancer treatment, chemical synthesis, and materials science. Surprisingly, a fundamental understanding of the molecular properties underlying these differences is lacking. Here, we report our study comparing the electronic structure and reactivity of closo-borate anions [closo-BnXn]2− (X = Cl, Br, I, n = 10, 11, 12 in all combinations) in the gas phase and in solution. We investigated the free dianions and the ion pairs [nBu4N]+[closo-BnXn]2− by gas phase anion photoelectron spectroscopy accompanied by theoretical investigations. Strong similarities in electronic structures for n = 10 and 11 were observed, while n = 12 clusters were different. A systematic picture of the development in electronic stability along the dimension X is derived. Collision induced dissociation shows that fragmentation of the free dianions is mainly dependent on the substituent X and gives access to a large variety of boron-rich molecular ions. Fragmentation of the ion pair depends strongly on n. The results reflect the high chemical stability of clusters with n = 10 and 12, while those with n = 11 are much more prone to dissociation. We bridge our study to the condensed phase by performing comparative electrochemistry and reactivity studies on closo-borates in solution. The trends found at the molecular level are also reflected in the condensed-phase properties. We discuss how the gas phase values allow evaluation of the influence of the condensed phase on the electronic stability of closo-borates. A synthetic method via an oxidation/chlorination reaction yielding [closo-B10Cl10]2− from highly chlorinated {closo-B11} clusters is introduced, which underlines the intrinsically high reactivity of the {closo-B11} cage.
Link: https://pubs.rsc.org/en/Content/ArticleLanding/2019/CP/C8CP05313H#!divAbstract