Just Published in Inorganic Chemistry
10.07.2023Isostructural Series of Ni(II), Pd(II), Pt(II), and Au(III) Azido Complexes with a N^C^N Pincer Ligand to Elucidate Trends in the iClick Reaction Kinetics and Structural Parameters of the Triazolato Products
Authors: Dominik Moreth, Gerald Hörner, Victoria V. L. Müller, Lucia Geyer, and Ulrich Schatzschneider*
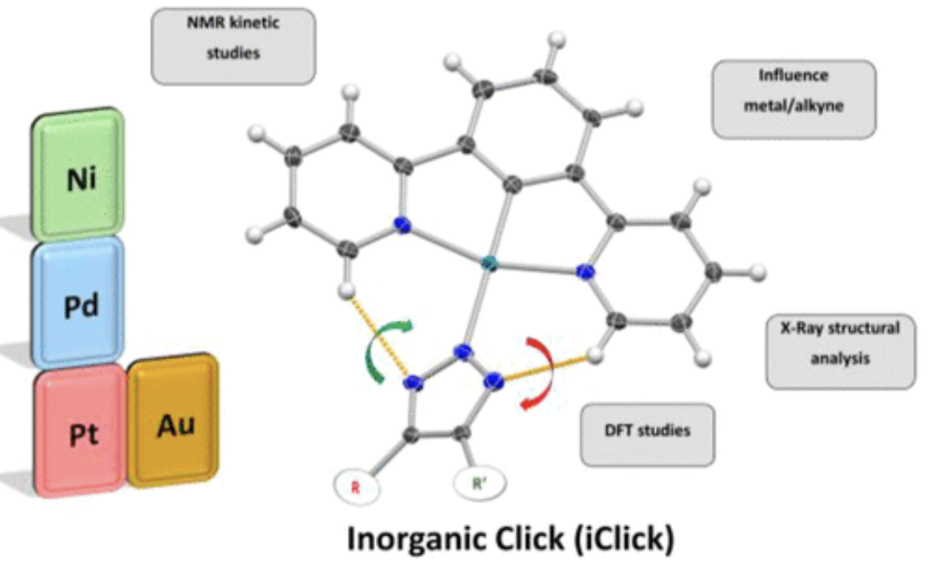
Abstract: An isoelectronic and isostructural series of cyclometalated azido complexes [M(N3)(dpb)] with M = Ni(II), Pd(II), Pt(II), and Au(III) based on the N^C^N pincer ligand 1,3-di(2-pyridyl)phenide (dpb) was characterized by X-ray diffraction analysis and investigated for reactivity in the iClick reaction with a wide range of internal and terminal alkynes by using 1H and 19F NMR spectroscopy. Reaction rate constants were found to increase with greater charge density in the order Ni(II) > Pd(II) > Pt(II) > Au(III). Terminal alkynes R–C≡C–R′ with strongly electron-withdrawing groups R and R′ exhibited faster kinetics than those with electron-donating substituents in the order CF3 > ketone > ester > H > phenyl ≫ amide, while R = CH3 resulted in complete loss of reactivity. Four symmetrical triazolato complexes [M(triazolatoCOOCH3,COOCH3)(dpb)] with M = Ni(II), Pd(II), Pt(II), and Au(III) as well as four nonsymmetrically substituted triazolato complexes [Pt(triazolatoR,R′)(dpb)] originating from terminal and internal alkynes were shown by X-ray crystal structure analysis to exclusively feature N2-coordination of the five-membered ring ligand. However, the Pt(II) triazolato complexes exist as a mixture of N1- and N2-coordinated species in solution. Torsion angles between the mean planes of the N^C^N pincer and the triazolato ligand increase from a nearly coplanar to a perpendicular arrangement when going from Au(III)/Pt(II)/Pd(II) to Ni(II), while different substituents R and R′ on the alkyne have no influence on the torsion angle and were rationalized by DFT calculations. Finally, a carbohydrate derivative obtained by glucuronic acid conjugation to methyl propiolate demonstrates the facile biofunctionalization of metal complexes via the iClick reaction.
Link: https://pubs.acs.org/doi/10.1021/acs.inorgchem.3c02122