Just Published in Chemistry - A European Journal
17.11.2016Cationic Bismuth Amides: Accessibility, Structure, and Reactivity
Authors: Hannah Dengel, Dr. Crispin Lichtenberg
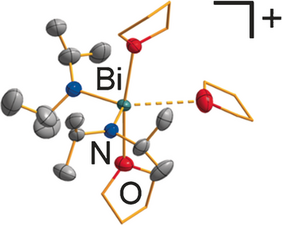
Abstract: The synthetic access to cationic bismuth compounds based on simple, monodentate, synthetically useful amido ligands, [Bi(NR2)2(L)n]+, has been investigated (R=Me, iPr, Ph; L=neutral ligand). With [BPh4]− as a counteranion, the formation of contact ion pairs and subsequent phenyl transfer from B to Bi is observed. An intermediate of this reaction, [Bi(NMe2)2(HNMe2)(BPh4)] (1), could be isolated and fully characterized. The use of a fluorinated tetraarylborate as a counteranion leads to more stable cationic bismuth amides. The solvent-separated ion pairs [Bi2(μ2-NMe2)2(NMe2)2(thf)6]2+ (4) and [Bi(NiPr2)2(thf)3]+ (5) were fully characterized, with [B(3,5-C6H3(CF3)2)4]−anions balancing the positive charge. The coordination chemistry, aggregation in solution, and spectroscopic features of these compounds were investigated. Compounds 4 and 5 show an increased reactivity towards diisopropylcarbodiimide compared to their neutral parent compounds. These reactions result in formation of the first cationic bismuth guanidinates. Characterization techniques include 1H, 11B, 13C, 15N, 19F, and 31P (VT)NMR and IR spectroscopy, single crystal X-ray diffraction analysis, and DFT calculations.
Link: http://onlinelibrary.wiley.com/doi/10.1002/chem.201604117/abstract