Just Published in Chemical Science
23.02.2023Nickel boryl complexes and nickel-catalyzed alkyne borylation
Authors: Lukas Tendera, Felipe Fantuzzi, Todd B. Marder and Udo Radius
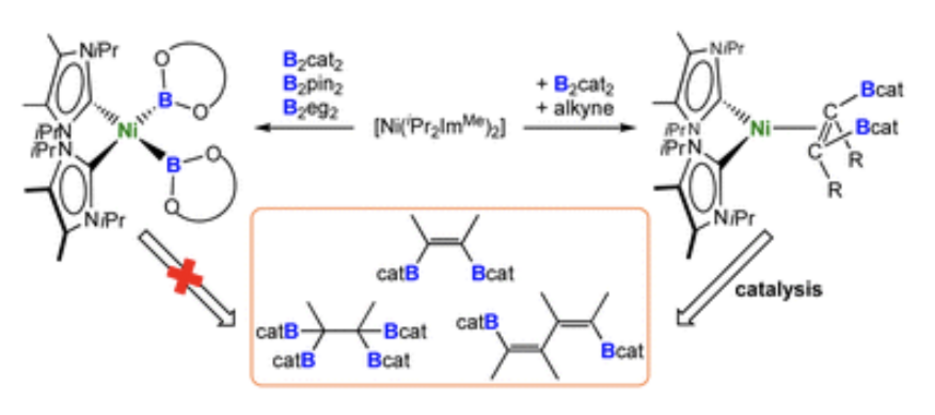
Abstract: The first nickel bis-boryl complexes cis-[Ni(iPr2ImMe)2(Bcat)2], cis-[Ni(iPr2ImMe)2(Bpin)2] and cis-[Ni(iPr2ImMe)2(Beg)2] are reported, which were prepared via the reaction of a source of [Ni(iPr2ImMe)2] with the diboron(4) compounds B2cat2, B2pin2 and B2eg2 (iPr2ImMe = 1,3-di-iso-propyl-4,5-dimethylimidazolin-2-ylidene; B2cat2 = bis(catecholato)diboron; B2pin2 = bis(pinacolato)diboron; B2eg2 = bis(ethylene glycolato)diboron). X-ray diffraction and DFT calculations strongly suggest that a delocalized, multicenter bonding scheme dictates the bonding situation of the NiB2 moiety in these square planar complexes, reminiscent of the bonding situation of “non-classical” H2 complexes. [Ni(iPr2ImMe)2] also efficiently catalyzes the diboration of alkynes using B2cat2 as the boron source under mild conditions. In contrast to the known platinum-catalyzed diboration, the nickel system follows a different mechanistic pathway, which not only provides the 1,2-borylation product in excellent yields, but also provides an efficient approach to other products such as C–C coupled borylation products or rare tetra-borylated compounds. The mechanism of the nickel-catalyzed alkyne borylation was examined by means of stoichiometric reactions and DFT calculations. Oxidative addition of the diboron reagent to nickel is not dominant; the first steps of the catalytic cycle are coordination of the alkyne to [Ni(iPr2ImMe)2] and subsequent borylation at the coordinated and, thus, activated alkyne to yield complexes of the type [Ni(NHC)2(η2-cis-(Bcat)(R)CC(R)(Bcat))], exemplified by the isolation and structural characterization of [Ni(iPr2ImMe)2(η2-cis-(Bcat)(Me)CC(Me)(Bcat))] and [Ni(iPr2ImMe)2(η2-cis-(Bcat)(H7C3)CC(C3H7)(Bcat))].
Link: https://pubs.rsc.org/en/content/articlelanding/2023/sc/d2sc04690c