Just Published in Chemical Communications
31.10.2016Cyclisation of biscarbenoids – a novel mode of cyclobutadiene stabilisation
Authors: Rüdiger Bertermann, Holger Braunschweig,* Mehmet Ali Celik, Theresa Dellermann, Hauke Kelch
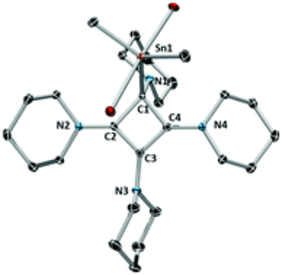
Abstract: The reaction of the vicinal biscarbenoid Pip–CC–Pip with dimethyltin dichloride yields a unique tetraamino-substituted cyclobutadienyl system featuring a dative C–Sn interaction. DFT investigation of the reaction mechanism revealed that coordination of the stannyl fragment to the nucleophilic carbon leads to a metal-stabilised zwitterion, allowing for [2+2] cycloaddition under thermal conditions. The compound features a homoaromatic π-system comprising the three sp2-hybridised carbon atoms of the four-membered ring as a consequence of charge separation.
Link: http://pubs.rsc.org/en/Content/ArticleLanding/2016/CC/C6CC07741B#