Synthetic Fluorine Chemistry
Electrochemical Fluorination
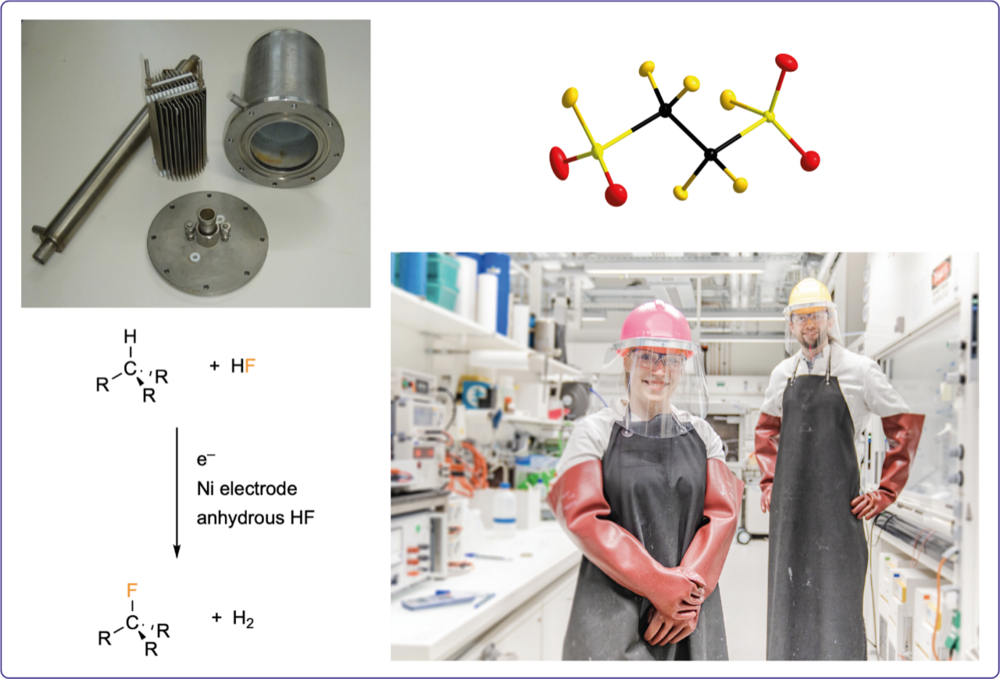
The electrochemical fluorination (ECF, Simons Process) in anhydrous HF is a versatile tool for the preparation of poly- and perfluorinated compounds. We employ this sustainable technique on a multigram to kilogram scale for the preparation of highly fluorinated compounds such as perfluoroalkylimides, perfluoroalkylphosphoranes, and fluoroborates. These compounds are key starting materials employed in a number of different research projects often in collaboration with partners from industry.
Publications on this topic:
Innovative Syntheses of Cyano(fluoro)borates: Catalytic Cyanation, Electrochemical and Electrophilic Fluorination
M. Drisch, L. A. Bischoff, J. A. P. Sprenger, P. T. Hennig, R. Wirthensohn, S. Z. Konieczka, M. Hailmann, N. V. Ignat'ev, M. Finze, Chem. Eur. J. 2020, 26, 11625–11633.
Verfahren zur Herstellung von Verbindungen mit Monofluorotricyanoborat-Anionen
J. A. P. Sprenger, L. A. Bischoff, M. Drisch, L. Herkert, M. Finze, H. Willner, E. Bernhardt, N. Ignatyev, M. Schulte, Merck Patent GmbH, WO2016058665, 2016.
Direct Fluorination
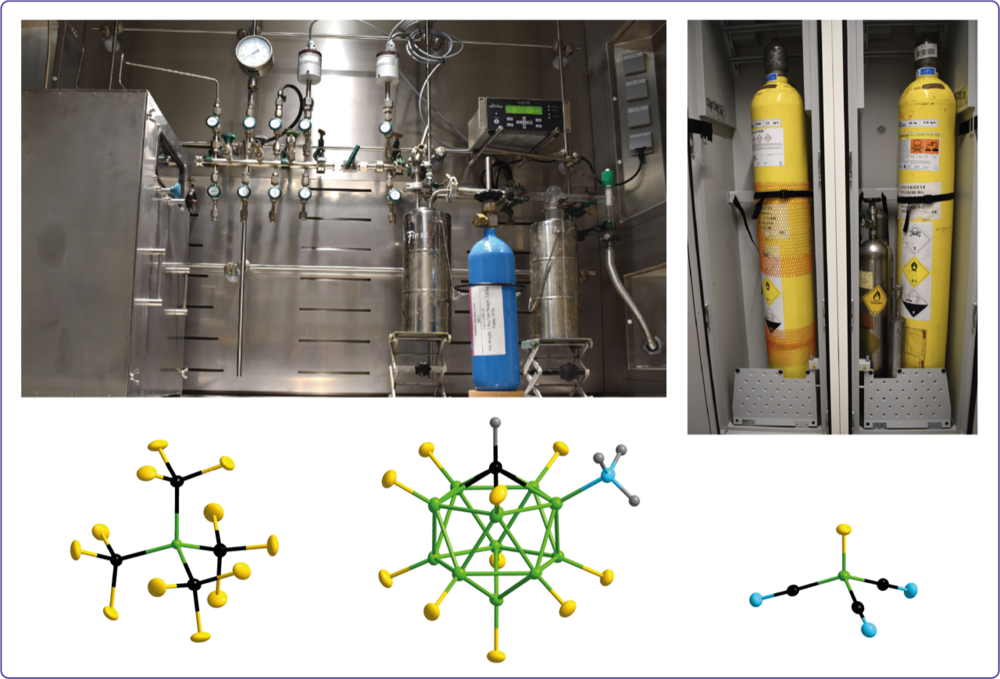
A broad variety of fluorinating reagents including elemental fluorine (F2), chlorine mono- and trifluoride (ClF and ClF3), sulfur tetrafluoride (SF4), and xenon difluoride (XeF2) are used for the synthesis of partially and fully fluorinated molecules. These molecules serve as building blocks for synthetic tasks and materials applications.
Publications on this topic:
Polyfluorinated carba-closo-dodecaboranes with amino and ammonio substituents bonded to boron
S. Z. Konieczka, M. Drisch, K. Edkins, M. Hailmann, M. Finze, Dalton Trans. 2015, 44, 19576–19586.
Mechanistic Study on the Fluorination of K[B(CN)4] with ClF Enabling the High Yield and Large Scale Synthesis of K[B(CF3)4] and K[(CF3)3BCN]
E. Bernhardt, M. Finze, H. Willner, Inorg. Chem. 2011, 50, 10268–10273.
Innovative Syntheses of Cyano(fluoro)borates: Catalytic Cyanation, Electrochemical and Electrophilic Fluorination
M. Drisch, L. A. Bischoff, J. A. P. Sprenger, P. T. Hennig, R. Wirthensohn, S. Z. Konieczka, M. Hailmann, N. V. Ignat'ev, M. Finze, Chem. Eur. J. 2020, 26, 11625–11633.
Perfluoroalkylnitrogen Chemistry
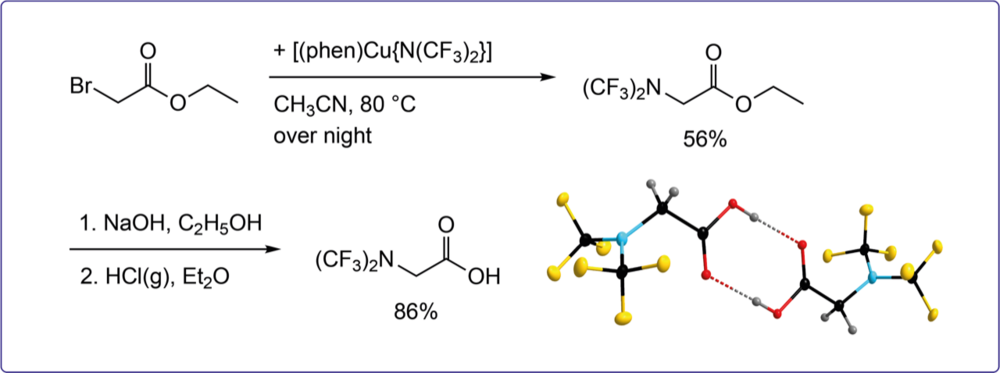
Perfluoroalkylnitrogen compounds are of increasing interest for the development of pharmaceuticals, agrochemicals, and new materials. We are interested in new strategies for the preparation of bis(trifluoromethyl)nitrogen compounds and related molecules. The syntheses rely on (CF3)2N–SO2CF3 as key starting compound that is synthesized via electrochemical fluorination (ECF).
Publications on this topic:
Stable and Storable N(CF3)2 Transfer Reagents
L. N. Schneider, E.-M. Tanzer Krauel, C. Deutsch, K. Urbahns, T. Bischof, K. A. M. Maibom, J. Landmann, F. Keppner, C. Kerpen, M. Hailmann, L. Zapf, T. Knuplez, R. Bertermann, N. V. Ignat’ev, M. Finze, Chem. Eur. J. 2021, 27, 10973–10978.
Transfer of Fluorinated Substituents
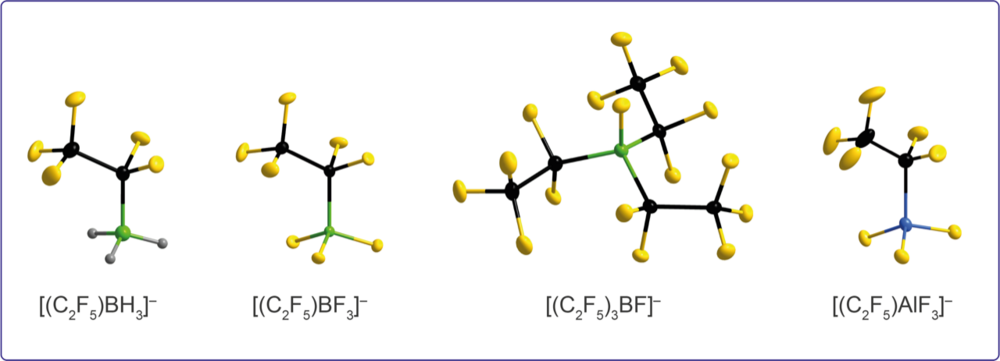
Fluoroalkylation reactions are key synthetic steps that provide access to precursors for e.g. the design of weakly coordinating anions and strong Lewis acids.
Publications on this topic:
Pentafluoroethylaluminates: A Combined Synthetic, Spectroscopic, and Structural Study
L. A. Bischoff, J. Riefer, R. Wirthensohn, T. Bischof, R. Bertermann, N. V. Ignat’ev, M. Finze, Chem. Eur. J. 2020, 26, 13615–13620.
The pentafluoroethyltrihydridoborate anion: from shock sensitive salts to stable room temperature ionic liquids
P. T. Hennig, J. A. P. Sprenger, L. N. Schneider, N. V. Ignat’ev, M. Finze, Chem. Commun. 2019, 55, 6110–6113.
Convenient synthesis of perfluoroalkyltrifluoroborates
J. A. P. Sprenger, C. Kerpen, N. Ignat'ev, M. Finze, J. Fluorine Chem. 2018, 206, 54–60.