Functionalized Boron Clusters
Cross-Coupling Reactions of Iodinated Boron Clusters
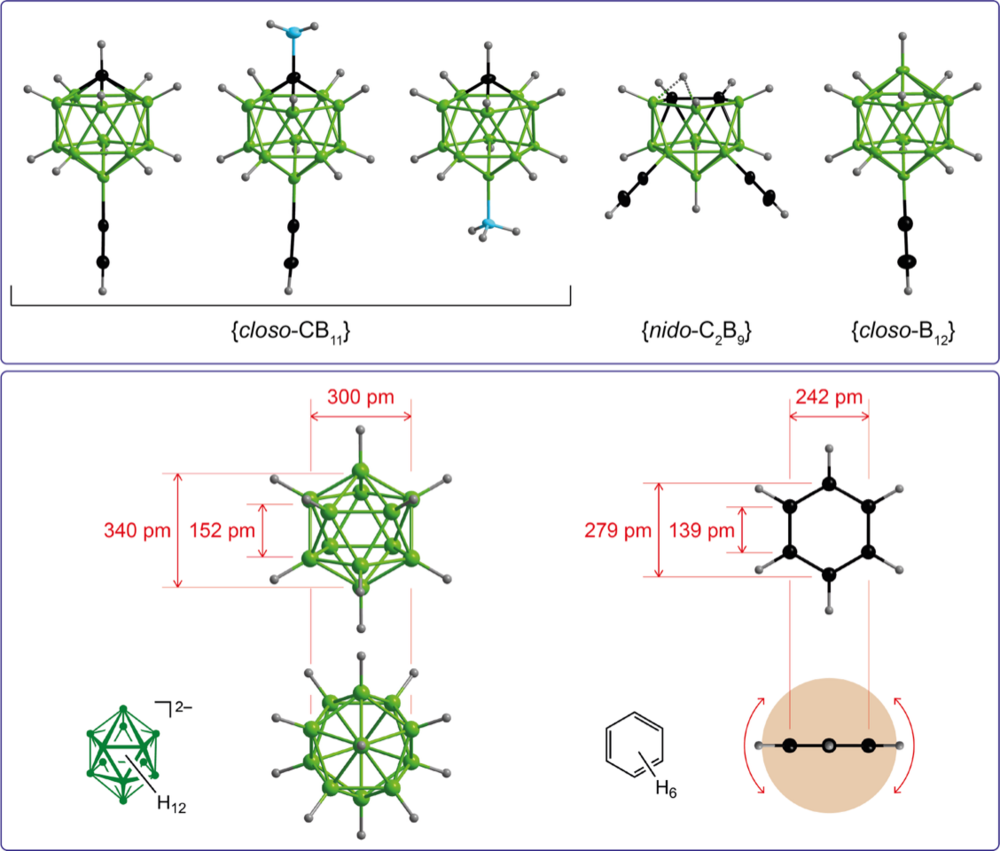
Cross-coupling reactions starting from mono- and polyiodinated boron clusters is a versatile method for the introduction of functional groups at boron cages. We are developing new catalytic methods towards neutral and anionic boron cages with BCluster–C and BCluster–N bonds that are easy-to-perform and scalable. The boron cages with functional groups are of interest as 3-dimensional substitutes for related benzenes.
Publications on this topic:
Synthesis, Characterization, and Selected Properties of 7- and 12-Ammoniocarba-closo-dodecaboranes
S. Z. Konieczka, A. Himmelspach, M. Hailmann, M. Finze, Eur. J. Inorg. Chem. 2013, 134–146.
Difunctionalized {closo-1-CB11} Clusters: 1- and 2-Amino-12-ethynylcarba-closo-dodecaborates
M. Hailmann, L. Herkert, A. Himmelspach, M. Finze, Chem. Eur. J. 2013, 19, 15745–15758.
Carba-closo-dodecaborate Anions with Two Functional Groups: [1-R- 12-HC≡C-closo-1-CB11H10]− (R = CN, NC, CO2H, C(O)NH2, NHC(O)H)
M. Hailmann, S. Z. Konieczka, A. Himmelspach, J. Löblein, G. J. Reiss, M. Finze, Inorg. Chem. 2014, 53, 9385–9399.
Polyhalogenated & Cyanated Anionic Boron Clusters
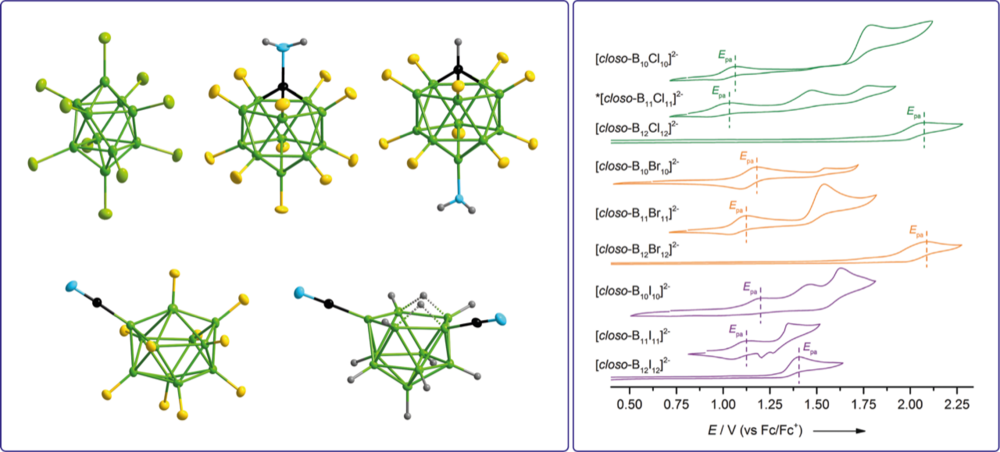
Novel boron clusters with halogen and related pseudohalogen substituents are prepared as versatile building blocks that comprise a weakly coordinating backbone and have one or more functional groups, which enable a tailored modification. These boron cluster-based building blocks are used in different fields of synthetic chemistry and materials applications, e.g. ionic liquids, ligands for coordination chemistry, and weakly coordinating anions. The syntheses include novel cluster Aufbau and contraction reactions as well as detailed studies on the chemical, electrochemical, and thermal stabilities.
Publications on this topic:
Properties of perhalogenated {closo-B10} and {closo-B11} multiply charged anions and a critical comparison with {closo-B12} in the gas and the condensed phase
J. Warneke, S. Z. Konieczka, G.-L. Hou, E. Aprà, C. Kerpen, F. Keppner, T. C. Schäfer, M. Deckert, Z. Yang, E. J. Bylaska, G. E. Johnsson, J. Laskin, S. X. Xantheas, X.-B. Wang, M. Finze, Phys. Chem. Chem. Phys. (PCCP) 2019, 21, 5903–5915.
Stepwise Introduction of Cyano Groups into nido- and closo-Undecaborate Clusters
S. Z. Konieczka, F. Schlüter, C. Sindorf, C. Kerpen, E. Bernhardt, M. Finze, Chem. Eur. J. 2018, 24, 3528–3538.
Polyfluorinated carba-closo-dodecaboranes with amino and ammonio substituents bonded to boron
S. Z. Konieczka, M. Drisch, K. Edkins, M. Hailmann, M. Finze, Dalton Trans. 2015, 44, 19576–19586.
Ligand Design with Anionic Boron Clusters
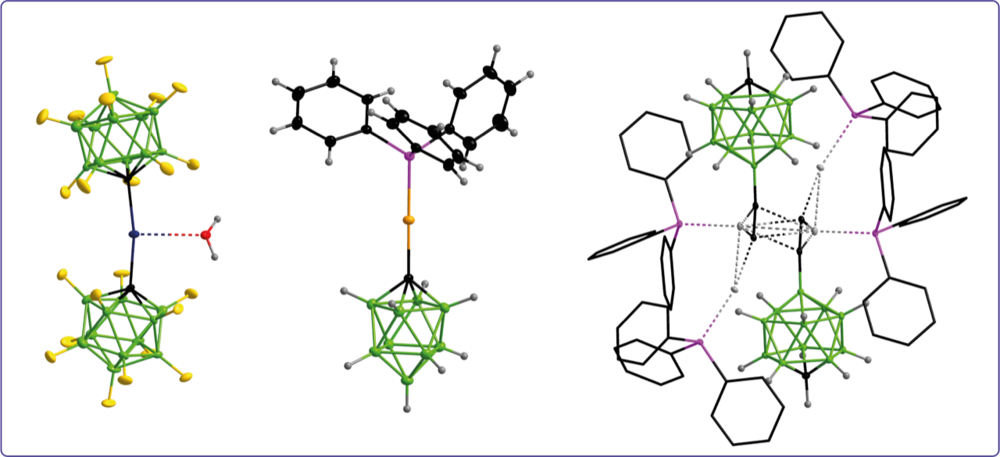
Boron clusters are employed as building blocks for the design of ligands for coordination chemistry. The boron cages are either directly coordinated to the metal center or are bonded to the ligand fragment that binds to the metal center. The choice of boron cluster and the substitution scheme enable a targeted ligand design and modulation of complex properties.
Publications on this topic:
Carba-closo-dodecaboranylethynyl ligands facilitating luminescent reversed charge-transfer excited states in gold/silver complexes
M. Hailmann, B. Hupp, A. Himmelspach, F. Keppner, P. T. Hennig, R. Bertermann, A. Steffen, M. Finze, Chem. Commun. 2019, 55, 9351–9354.
Unprecedented Efficient Structure Controlled Phosphorescence of Silver(I) Clusters Stabilized by Carba-closo-dodecaboranylethynyl Ligands
M. Hailmann, N. Wolf, R. Renner, T. C. Schäfer, B. Hupp, A. Steffen, M. Finze, Angew. Chem. Int. Ed. 2016, 55, 10507–10511.
Anionic Gold(I) Complexes—Twelve- and Ten-Vertex Monocarba-closo-borate Anions with Carbon–Gold σ Bonds
M. Finze, J. A. P. Sprenger, Chem. Eur. J. 2009, 15, 9918–9927.
Carboranyl-Substituents: Modulation of Reactivity & Electronic Properties
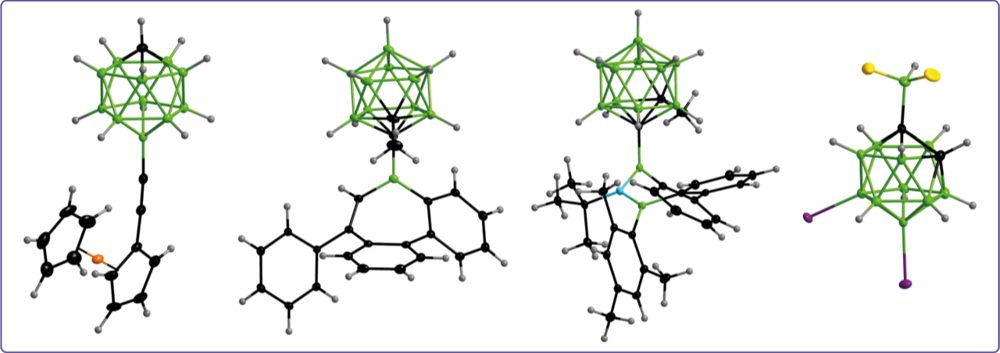
Carboranyl substituents reveal tunable properties that can be adjusted by the choice of the boron cage and the substitution scheme at the cluster. Currently, our research focusses on ortho-carboranyl substituents that act as electron-withdrawing and electronically active substituents.
Publications on this topic:
Alkene insertion reactivity of a o-carboranyl- substituted 9-borafluorene
T. Bischof, X. Guo, I. Krummenacher, L. Beßler, Z. Lin, M. Finze, H. Braunschweig, Chem. Sci. 2022, 13, 7492–7497.
Backbone-controlled LUMO energy induces intramolecular C–H activation in ortho-bis-9-borafluorene-substituted phenyl and o-carboranyl compounds leading to novel 9,10- diboraanthracene derivatives
J. Krebs, A. Häfner, S. Fuchs, X. Guo, F. Rauch, A. Eichhorn, I. Krummenacher, A. Friedrich, L. Ji, M. Finze, Z. Lin, H. Braunschweig, T. B. Marder, Chem. Sci. 2022, 13, 14165–14178.
Thermodynamic equilibrium between locally excited and charge-transfer states through thermally activated charge transfer in 1-(pyren-20- yl)-o-carborane
L. Ji, S. Riese, A. Schmiedel, M. Holzapfel, M. Fest, J. Nitsch, B. F. E. Curchod, A. Friedrich, L. Wu, H. H. Al Mamari, S. Hammer, J. Pflaum, M. A. Fox, D. J. Tozer, M. Finze, C. Lambert, T. B. Marder, Chem. Sci. 2022, 13, 5205–5219.