Nucleic Acid Assemblies
Nucleic acid merocyanine dimers
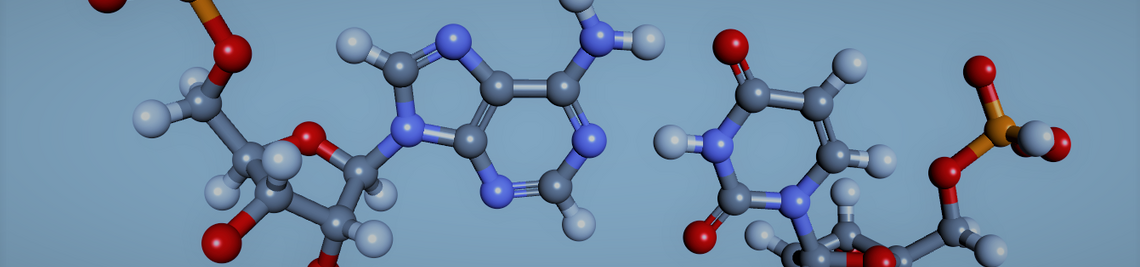
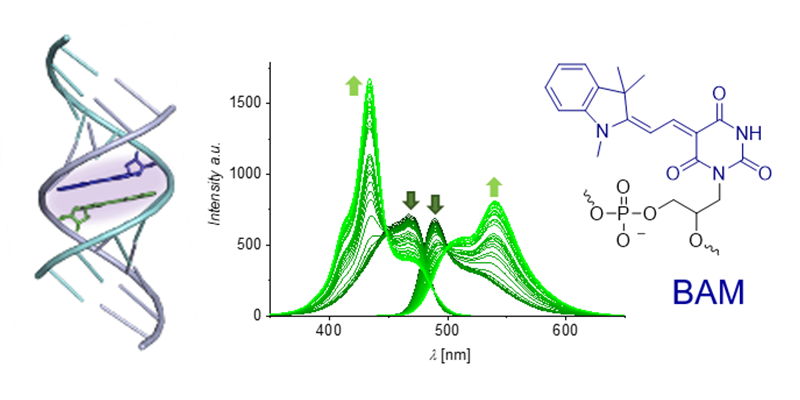
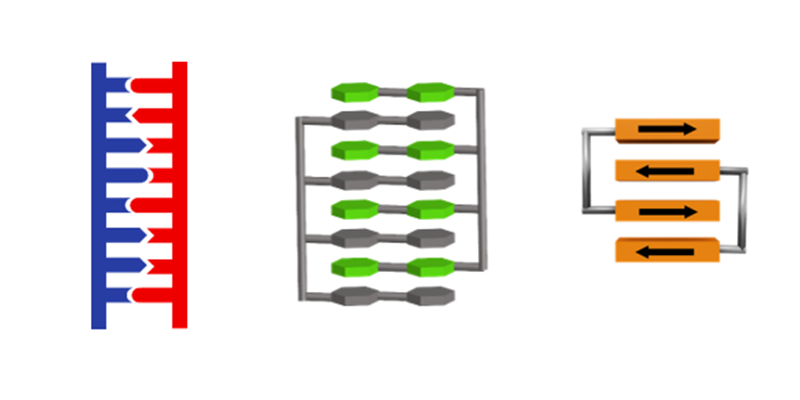
Exciton coupling between two or more chromophores in a specific environment is a key mechanism associated with color tuning and modulation of absorption energies. Barbituric acid merocyanine (BAM) nucleosides in synthetic nucleic acid nanostructures show exciton coupling that can be tuned by the double helix conformation. Duplexes with different backbone constitutions and geometries afford different mutual dye arrangements, leading to distinct optical signatures seen in absorption, CD and fluorescence spectroscopy.
J. Dietzsch, D. Bialas, J. Bandorf, F. Würthner, C. Höbartner
Tuning exciton coupling of merocyanine nucleoside dimers by RNA, DNA and GNA double helix conformations
Angew. Chem. Int. Ed. 2022.
J. Dietzsch, A. Jayachandran, S. Mueller, C. Höbartner and T. Brixner
Excitonic coupling of RNA-templated merocyanine dimer studied by higher-order transient absorption spectroscopy
Chem. Commun 2023, 59, 7395-7398
Bioorthogonal dipolar recognition
Inspired by natural Watson-Crick base pairing and genetic code expansion by hydrophobic base pairing, we explore dipolar and quadrupolar stacking interactions as bioorthogonal elements for the assembly of supramolecular nucleic acid structures.
H. Neitz, I. Bessi, V. Kachler, M. Michel, C. Höbartner
Tailored Tolane-Perfluorotolane Assembly as Supramolecular Base Pair Replacement in DNA
Angew. Chem. Int. Ed. 2022, e202214456 first published: November 7th.
Light-induced DNA crosslinking
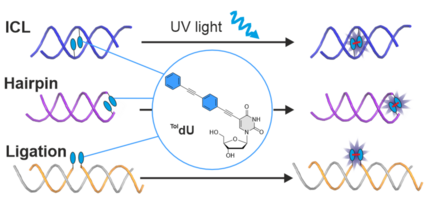
H. Neitz, I. Bessi, J. Kuper, C. Kisker, C. Höbartner
Programmable DNA Interstrand Crosslinking by Alkene–Alkyne [2 + 2] Photocycloaddition
J. Am. Chem. Soc. 2023, 145, 9428–9433
J. Kuper, T. Hove, S. Maidl, H. Neitz, F. Sauer, M. Kempf, T. Schroeder, E. Greiter, C. Höbartner & C. Kisker
XPD stalled on cross-linked DNA provides insight into damage verification
Nature Structural and Molecular Biology 2024, published online May 28