Description of Main Group Element Compounds
Description of Main Group Element Compounds
Investigation of Reaction Mechanisms
| The fast-developing field of main group chemistry brings forth more and more molecules and compounds rivaling transition metals with respect to their catalytic activity. [1] Whereas the reaction mechanisms of the latter are well understood for the most part, in the case of highly reactive main group element compounds the exact sequences of the underlying catalytic cycles are often still unclear. In our work, we use density functional theory expanded by thermochemistry corrections to investigate the hydroboration reaction via a reduced diboraanthracene (DBA) derivative as a case study. Free energy calculations revealed that the inclusion of the counter ion and implicit and explicit solvent effects are necessary to reproduce available experimental data (e.g. geometries found in the crystal, Figure 1) and to get a full picture of the energetic profile for this reaction, which was observed to be dependent on the counterions of the catalyst (Figure 2). This work is performed in cooperation with the Wagner group (Goethe-Universität Frankfurt), which performs the synthesis and the experimental measurements. [2] |
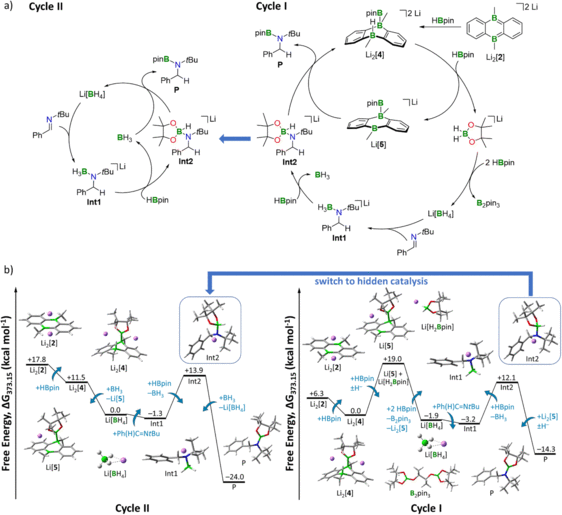
Figure 2: (a) (Right) Catalytic Cycle I (hydride shuttle: Li[H2Bpin]). (Left) Catalytic Cycle II, hidden catalysis (hydride shuttle: Li[BH4]). (b) (Right) Free energy diagram for catalytic Cycle I. (Left) Free energy diagram for hidden catalysis Cycle II including BH3·thf/Li[BH4]. Energies are referenced to the starting point of the respective cycle. Level of theory: SMD(solvent = THF)/ωB97XD/6-311++G(d,p) from optimized structures at SMD(solvent = THF)/ωB97XD/6-31+G(d,p). Explicit thf molecules are omitted for clarity.
[1] Nitrogen fixation and reduction at boron. M.-A. Légaré, G. Bélanger-Chabot, R. D. Dewhurst, E. Welz, I. Krummenacher, B. Engels, H. Braunschweig, Science 2018, 359, 896–900, http://doi.org/10.1126/science.aaq1684.
[2] Multifaceted behavior of a doubly reduced arylborane in B–H-bond activation and hydroboration catalysis. S. E. Prey, C. Herok, F. Fantuzzi, M. Bolte, H.-W. Lerner, B. Engels, M. Wagner, Chem. Sci. 2022, 14, 849-860, https://doi.org/10.1039/D2SC05518J.