AK Kurth
Supramolecular Materials (AK Kurth)
The working group of Prof. Kurth deals with self-assembling stimuli-responsive polymers based on metal ion coordination. The structures and the properties of the formed metallo-supramolecular polymers is investigated in detail. The work deals with the synthesis and self-assembly of the molecular building blocks and metal ions as well as with the structural and property investigations.
Self-assembly is a principle of materials science that is becoming increasingly important. In this process, the desired material is tailored by the appropriate structure of its building blocks and by their interactions with each other. Self-assembly is inspired by processes in nature and is based on weak interactions between the individual modules, such as hydrogen bonding, metal ion coordination or van der Waals forces. This results in stimuli-responsive materials that can change their structure and property by an external stimulus (temperature, pH, etc.) or adapt to the environment and have great potential for the development of new functional materials. The incorporation of metal ions into the structures leads to value-added functions that are of interest for magnetic, optical, and electronic applications.
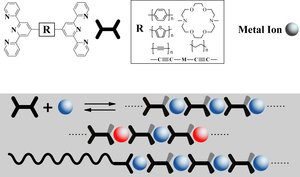
In AK Kurth, the main focus is on the self-assembly of supramolecular metal complex systems. In their preparation, for example, ditopic bis-pyridine ligands and kinetically labile transition metal ions such as Fe(II) or Ni(II) are used. In this process, so-called metallo-supramolecular polyelectrolytes (MEPEs) are formed (see Fig. 1). MEPEs can be incorporated into various systems such as mesoporous materials or coating films as electrochromic or magnetic materials to modify the properties of the substrate materials.
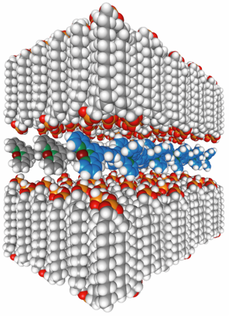
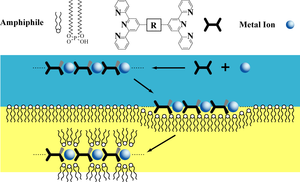
The counterions of MEPEs can be exchanged by ionic amphiphiles such as dehexadecyl phosphates (DHP), forming polyelectolyte-amphiphile complexes (PACs) with liquid crystalline properties (see Figs. 2 and 3). The modular structure of PACs from metal ions, ligands, and amphiphiles allows the structure and properties of PACs to be tailored by the choice of building blocks. Phase transitions of such amphiphilic mesophases can create mechanical stress in the system, leading to a change in the coordination geometry around the metal center. This can, for example, change the spin states of the metal ion and thus tune the electronic configuration of the metallo-supramolecule.
Work on such and similar supramolecular systems represents an extension of macromolecular chemistry beyond carbon-based polymers. This can vastly increase the structural diversity and property capacity of macromolecules.
Other work includes colloids and their manipulation in electric fields, as well as the interaction of thin films based on MEPE and cells.