Publications
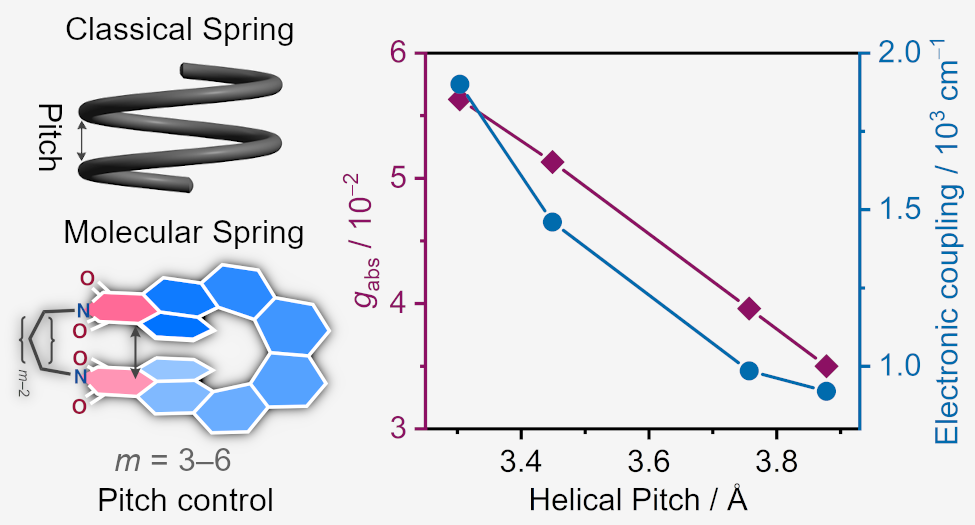
| 47 | Spring-Like Behavior in [8]Helicene Diimides: How Helical Pitch Governs Optical Anisotropy and Electronic Conjugation Saal, F.; Brancaccio, V.; Radacki, K.; Braunschweig, H.; Ravat, P.* Angew. Chem. Int. Ed. 2025. |
| 46 | Synthesis and Chiroptical Properties of Biscarbazole-Embedded Diaza[7]helicenes Upadhyay, G. M.; Swain, A.; Bhalodi, E. H.; Ravat, P.*; Bedekar, A. V.* J. Org. Chem. 2025. |
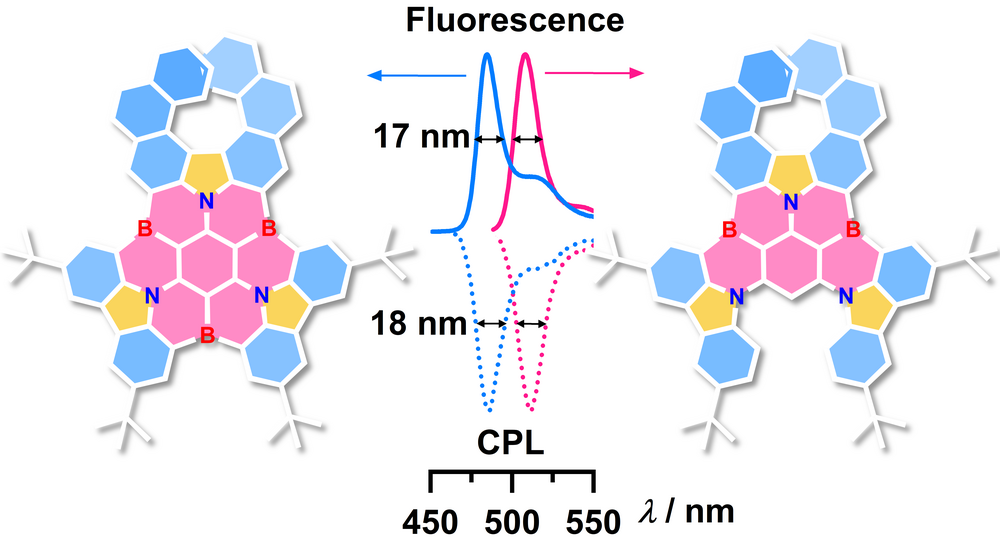
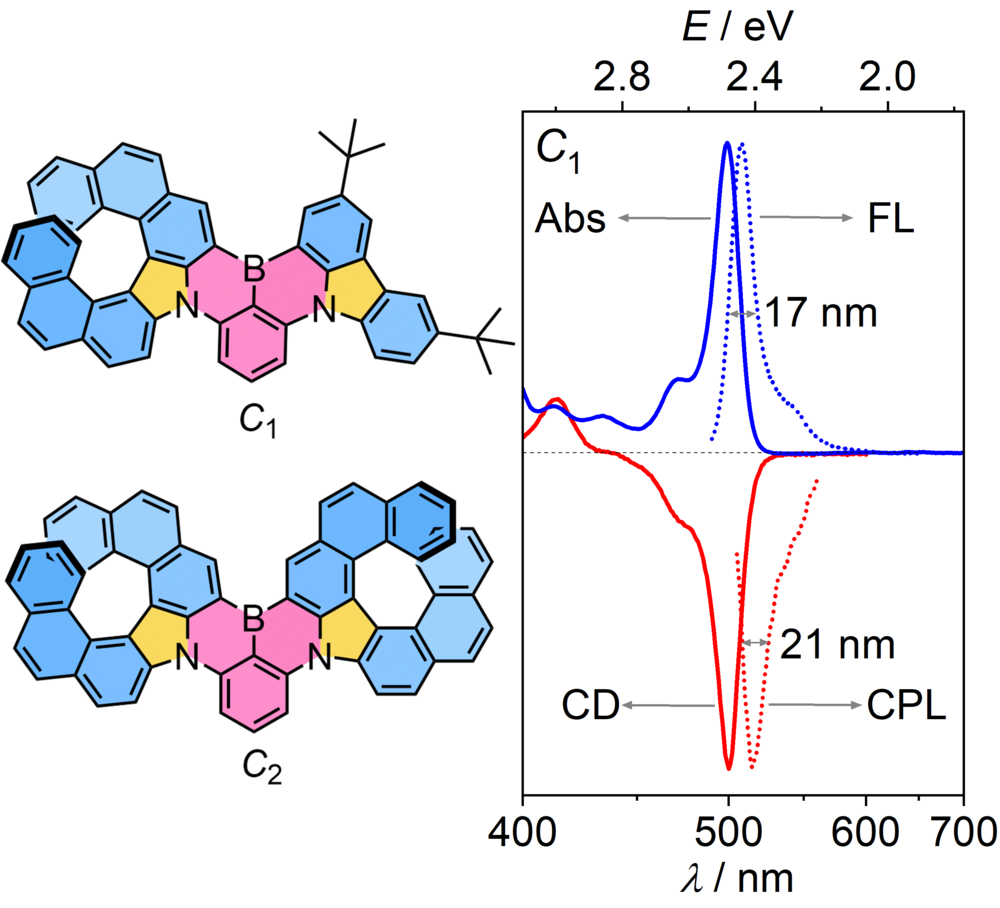
| 45 | Ultra-Narrowband Circularly Polarized Luminescence from Multiple 1,4-Azaborine-Embedded Helical Nanographenes Zhang, F.; Brancaccio, V.; Saal, F.; Deori, U.; Radacki, K.; Braunschweig, H.; Rajamalli, P.; Ravat, P.* J. Am. Chem. Soc. 2024. |
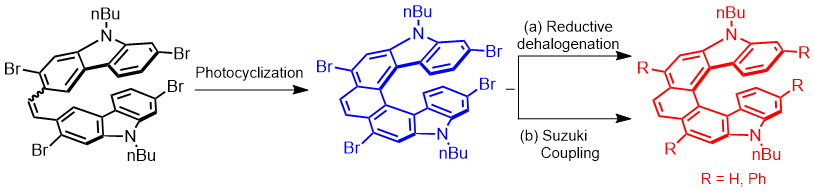
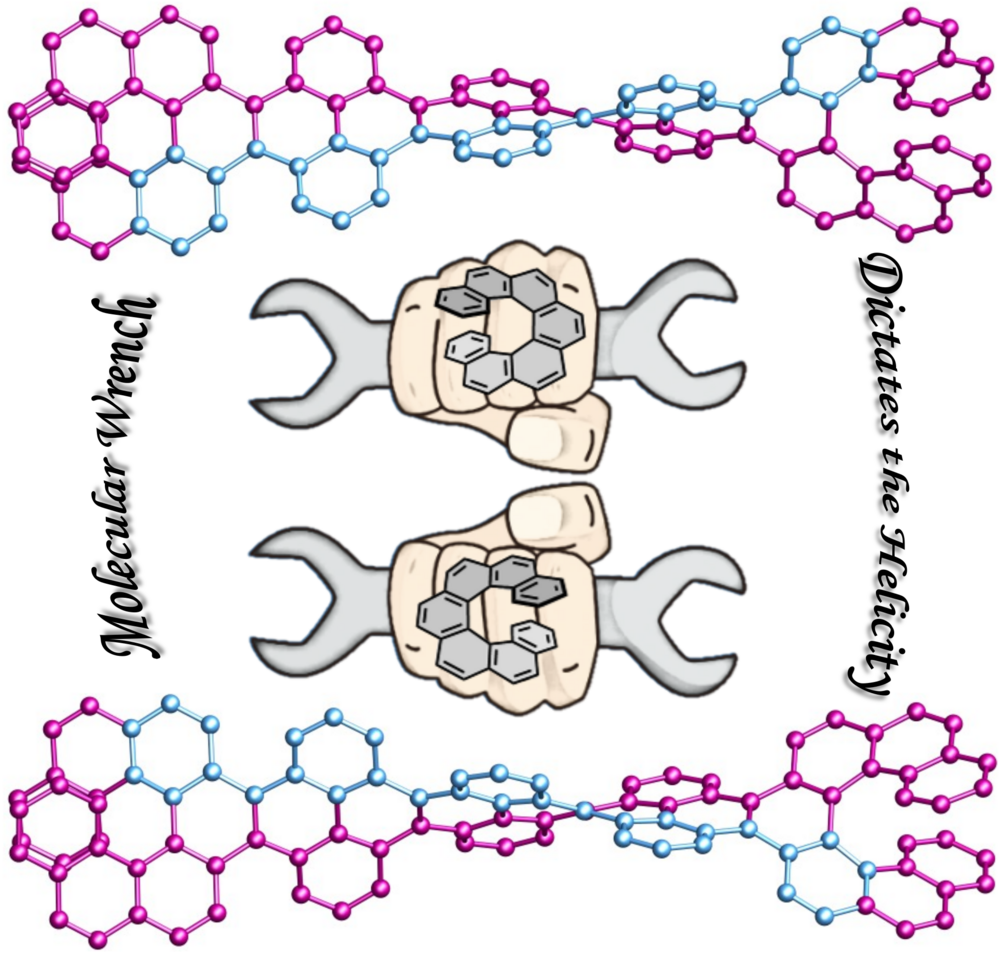
| 44 | Helically twisted nanoribbons via stereospecific annulative π-extension reaction employing [7]helicene as a molecular wrench Swain, A.; Radacki, K.; Braunschweig, H.; Ravat, P.* Chem. Sci. 2024. |
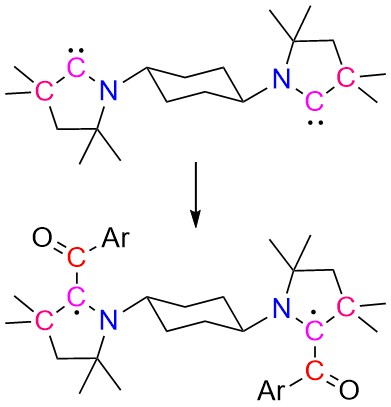
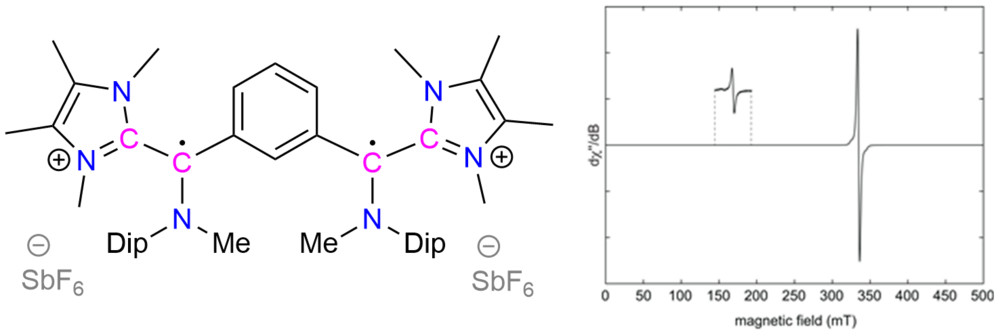
| 43 | Bis-[cyclic (alkyl)(amino)carbene]s derived diradicals Nayak, M. K.; Elvers, B. J.; Mehta, S.; Krummenacher, I.; Mondal, A.*; Braunschweig, H.*; Schulzke, C.*; Ravat, P.*; Jana, A.* Chem. Commun. 2024. |
| 42 | Aza[7]helicene Functionalized Triphenylmethyl Radicals with Circularly Polarized Doublet Emission Gross, M.; Zhang, F.; Arnold, M. E.; Ravat, P.*; Kuehne, A. J. C.* Adv. Optical Mater. 2024. DOI: 10.1002/adom.202301707 |
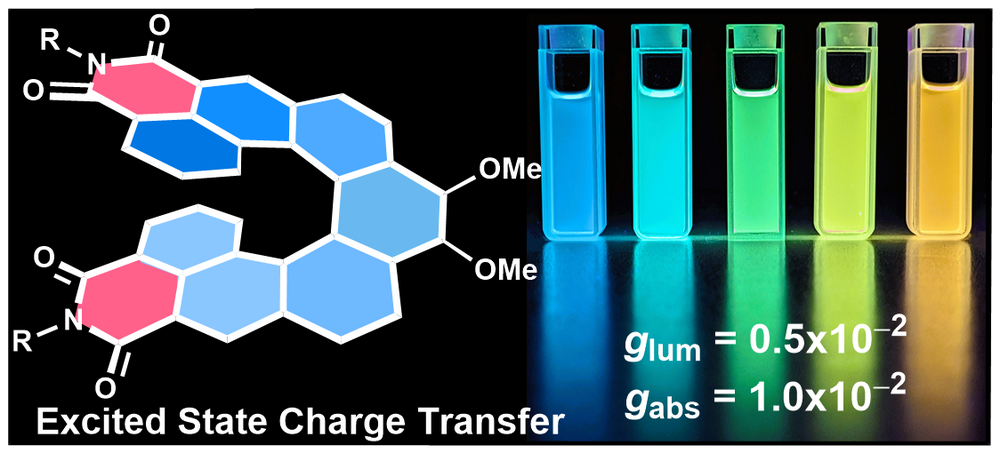
| 41 | Push–pull [7]helicene diimide: excited-state charge transfer and solvatochromic circularly polarised luminescence Saal, F.; Swain, A.; Schmiedel, A.; Holzapfel, M.; Lambert, C.; Ravat, P.* Chem. Commun. 2023. |
| 40 | Bis-Olefin Based Crystalline Schlenk Hydrocarbon Diradicals with a Triplet Ground State Saha, P.; Chrysochos, N.; Elvers, B. J.; Pätsch, S.; Uddin, S. I.; Krummenacher, I.; Nandeshwar, M.; Mishra, A.; Raman, K. V.; Rajaraman, G.*; Prabusankar, G.*; Braunschweig, H.*; Ravat, P. *; Schulzke, C.*; Jana, A.* Angew. Chem. Int. Ed., 2023, e202311868. DOI: 10.1002/anie.202311868 |
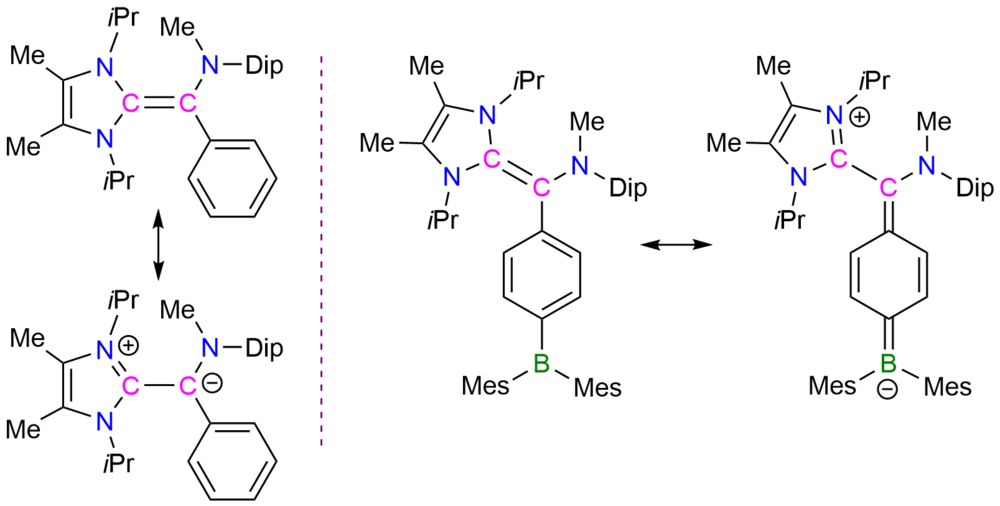
| 39 | Introducing an orthogonally polarized electron-rich alkene: synthesis of a zwitterionic boron-containing π-conjugated system Chrysochos, N.; Pätsch, S.; Elvers, B. J.; Krummenacher, I.; Muneshwar, N.; Ganesan, P.*; Braunschweig, H.*; Schulzke, C.*; Ravat, P.*; Jana, A.* Chem. Commun. 2023. DOI: 10.1039/D3CC03975G |
| 38 | Pyrene bridged double[7]helicene embedded with a heptagonal ring Swain, A.; Ravat, P.* Org. Chem. Front., 2023. Organic Chemistry Frontiers Emerging Investigator Series |
| 37 | Dimethylnonacethrene – en route to a magnetic switch Chem. Commun., 2023. Chemical Communications HOT Articles 2023 |
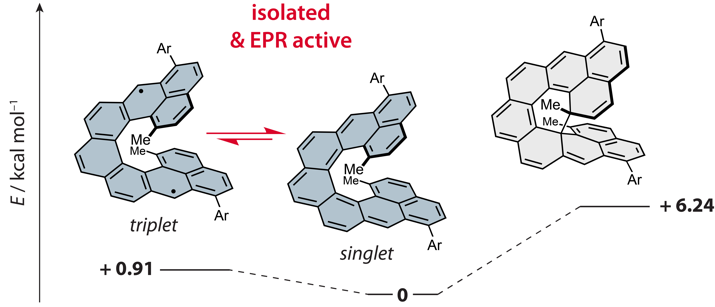
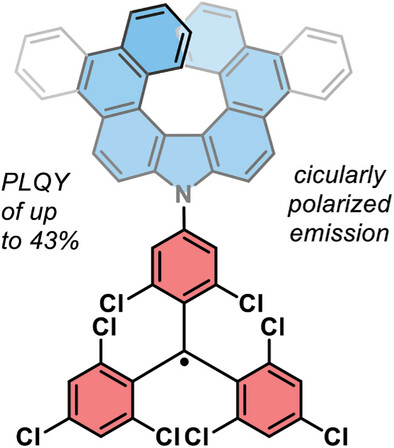
| 36 | Efficient Narrowband Circularly Polarized Light Emitters Based on 1,4-B,N-embedded Rigid Donor–Acceptor Helicenes Angew. Chem. Int. Ed., 2023. |
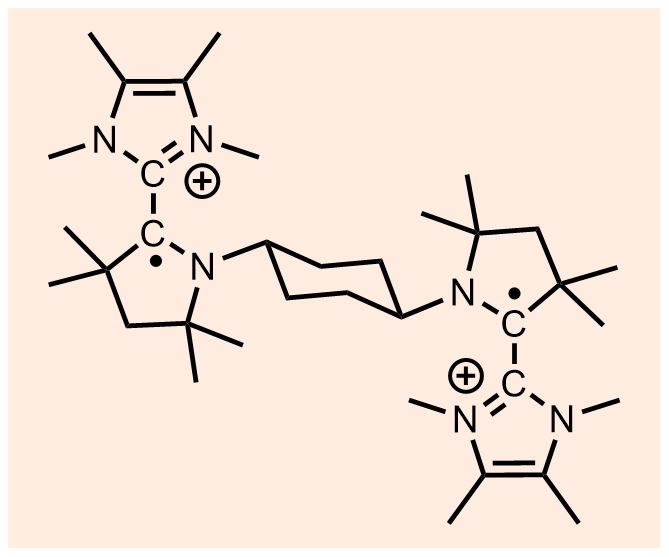
| 35 | A bis-NHC-CAAC dimer derived dicationic diradical Nayak, M. K.; Sarkar, P.; Elvers, B. J.; Mehta, S.; Zhang, F. M.; Chrysochos, N. D.; Krummenacher, I.; Vijayakanth, T.; Narayanan, R. S.; Dolai, R.; Roy, B. M.; Malik, V. M.; Rawat, H.; Mondal, A.*; Boomishankar, R.*; Pati, S. K.*; Braunschweig, H.*; Schulzke, C.*; Ravat, P.*; Jana, A.* Chem. Sci. 2022. Highlight in ChemistryViews. |
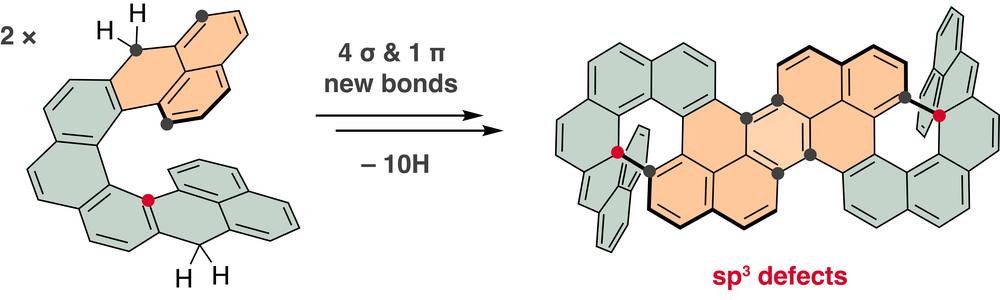
| 34 | Nonacethrene Unchained: A Cascade to Chiral Contorted Conjugated Hydrocarbon with Two sp3-Defects Čavlović D.; Häussinger D.; Blacque O.; ; Ravat, P.*; Juríček M.* JACS Au, 2022, 2, 1616–1626. ACS Editors’ Choice |
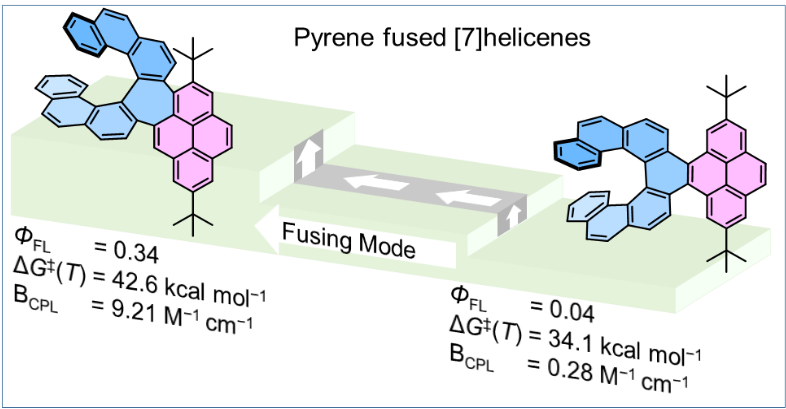
| 33 | Pyrene-Fused [7]Helicenes Connected via Hexagonal and Heptagonal Rings: Stereospecific Synthesis and Chiroptical Properties Swain, A.; Radacki, K.; Holger Braunschweig, H.; Ravat, P.* J. Org. Chem. 2022, 87, 993–1000. |
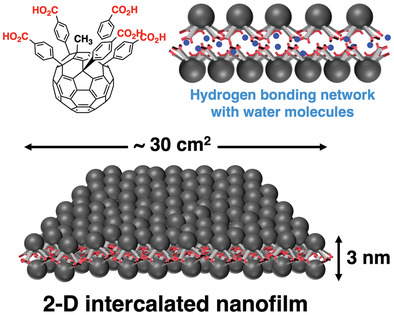
| 32 | De Novo Synthesis of Free-Standing Flexible 2D Intercalated Nanofilm Uniform over Tens of cm2 Ravat, P.; Uchida, H.; Sekine, R.; Kamei, K.; Yamamoto, A.; Konovalov, O.; Tanaka, M.; Yamada, T.; Harano, K.; Nakamura, E. Adv. Mater. 2022, 2106465. Special Issue: Organic Semiconductors |
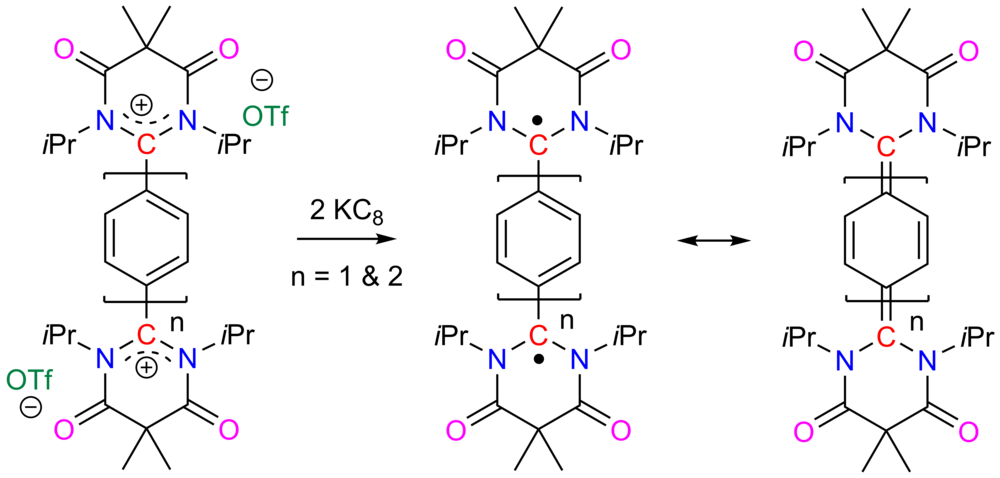
| 31 | Diamidocarbene-Based Thiele and Tschitschibabin Hydrocarbons: Carbonyl Functionalized Kekulé Diradicaloids Maiti, A.; Sobottka, S.; Chandra, S.; Jana, D.; Ravat, P.*; Sarkar, B.*; Jana, A.* J. Org. Chem. 2021, 86, 16464–16472. |
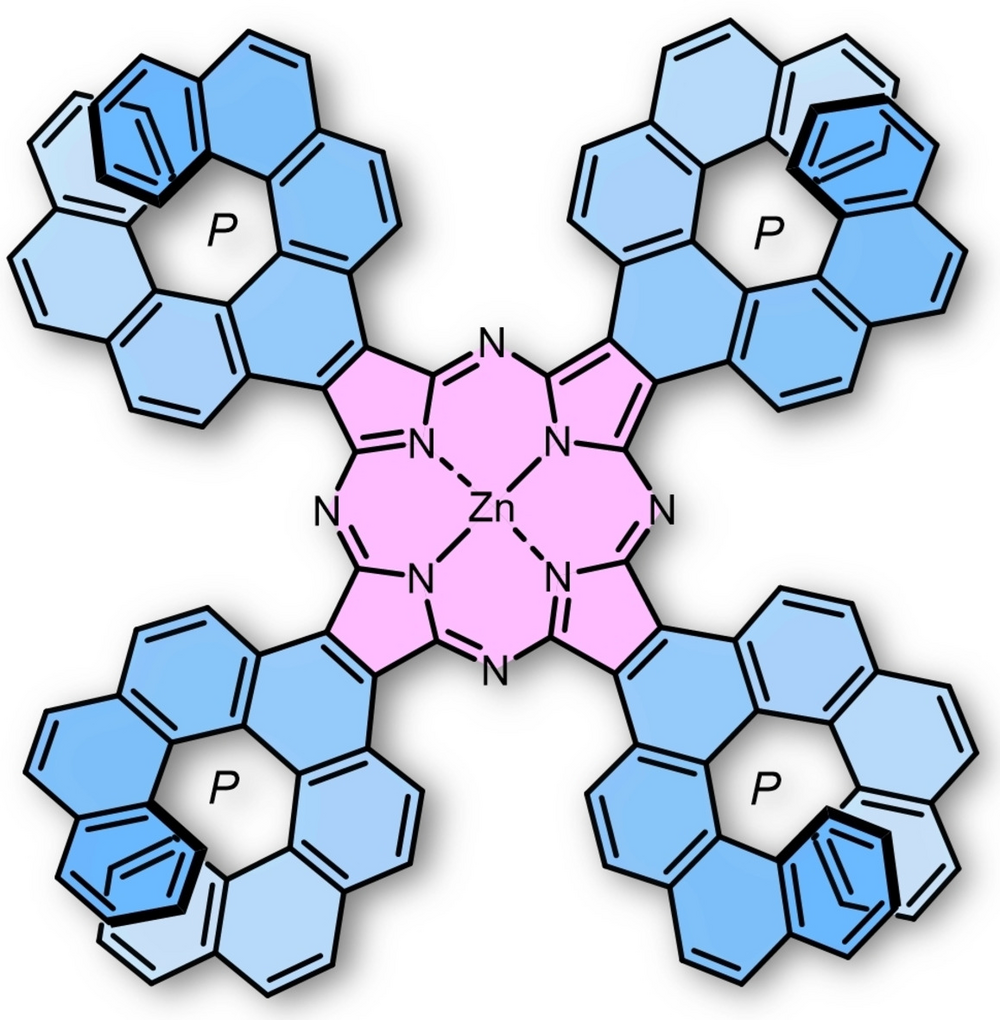
| 30 | Zinc-[7]helicenocyanine and Its Discrete π-Stacked Homochiral Dimer Zhang, F.; Radacki, K.; Braunschweig, H.; Lambert, C.; Ravat, P.* Angew. Chem. Int. Ed. 2021, 60, 23656–23660. |
| 29 | Imide-Functionalized Helical PAHs: A Step towards New Chiral Functional Materials Saal, F.; Ravat, P.* SynLett, 2021, 32, 1879–1890. |
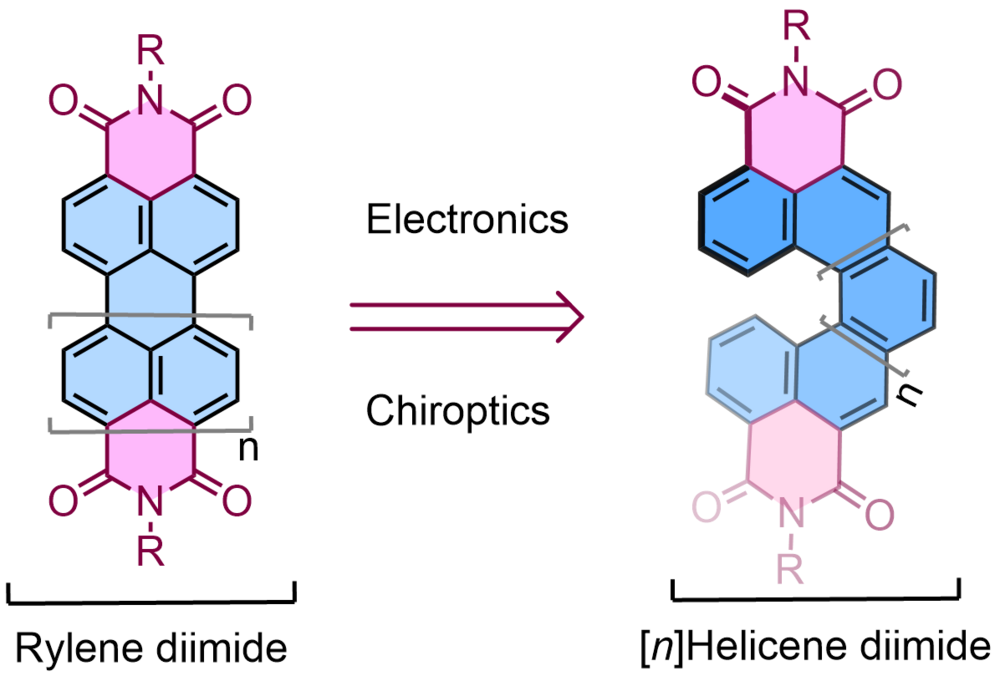
| 28 | Anionic Boron- and Carbon-Based Hetero-Diradicaloids Spanned by a p-Phenylene Bridge Maiti, A.; Zhang, F.; Krummenacher, I.; Bhattacharyya, M.; Mehta, S.; Moos, M.; Lambert, C.; Engels, B.*; Mondal, A.*; Braunschweig, H.*; Ravat, P.*; Jana, A.* J. Am. Chem. Soc. 2021, 143, 10, 3687–3692. DOI: 10.1021/jacs.0c12624 |
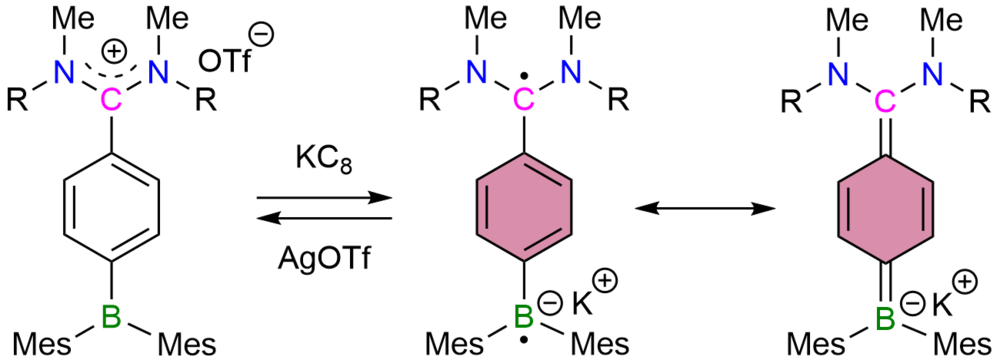
| 27 | Nano- and Microspheres Containing Inorganic and Biological Nanoparticles: Self-Assembly and Electron Tomographic Analysis Sekine, R.; Ravat, P.; Yanagisawa, H.; Liu, C.; Kikkawa, M.; Harano, K.; Nakamura, E. J. Am. Chem. Soc. 2021, 143, 2822–2828. DOI: 10.1021/jacs.0c11944 |
| 26 | C2- and C1-Symmetric Configurationally Stable Pyrene-Fused [5]Helicenes Connected via Hexagonal and Heptagonal Rings Swain, A.; Kolanji, K.; Christoph, S.; Ravat, P.* Org. Lett. 2021, 23, 1339–1343. |
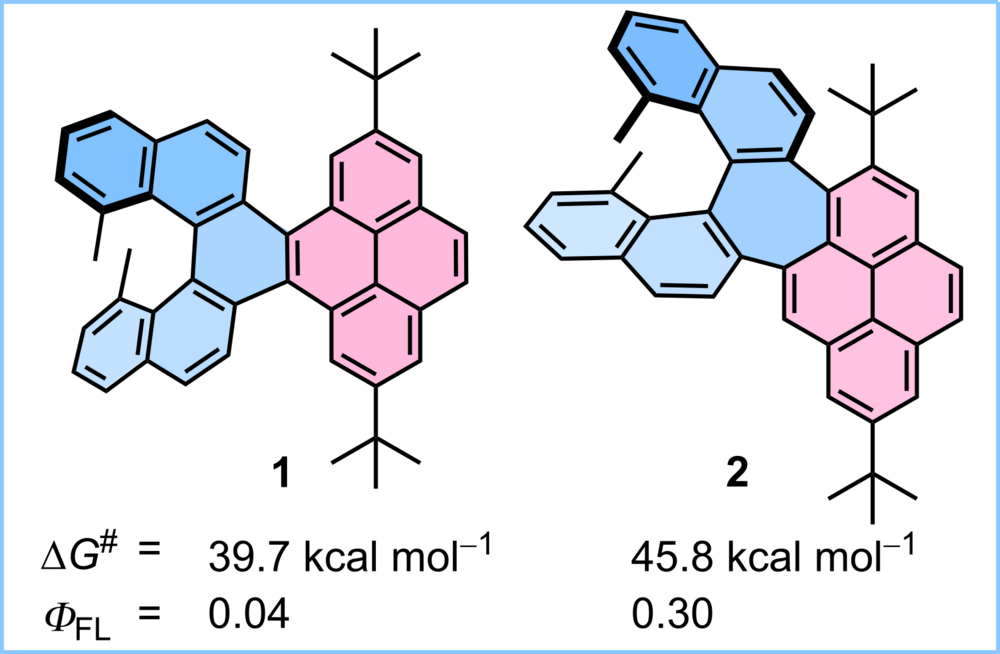
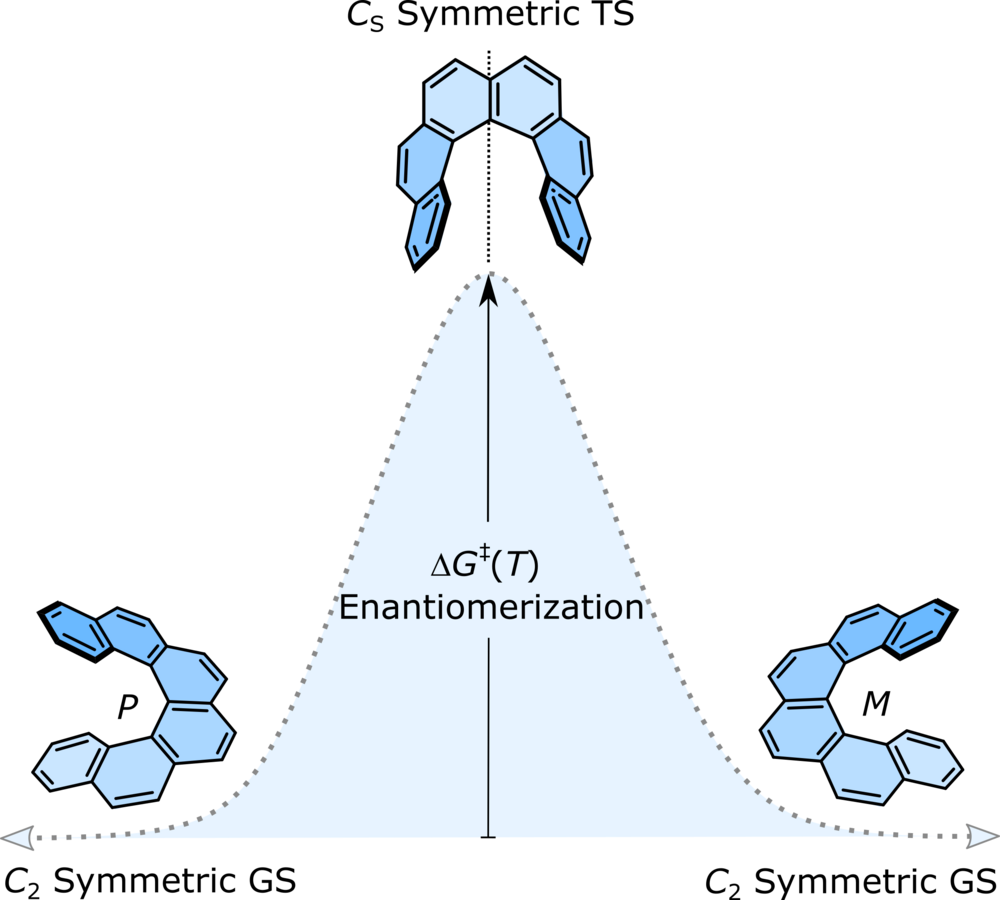
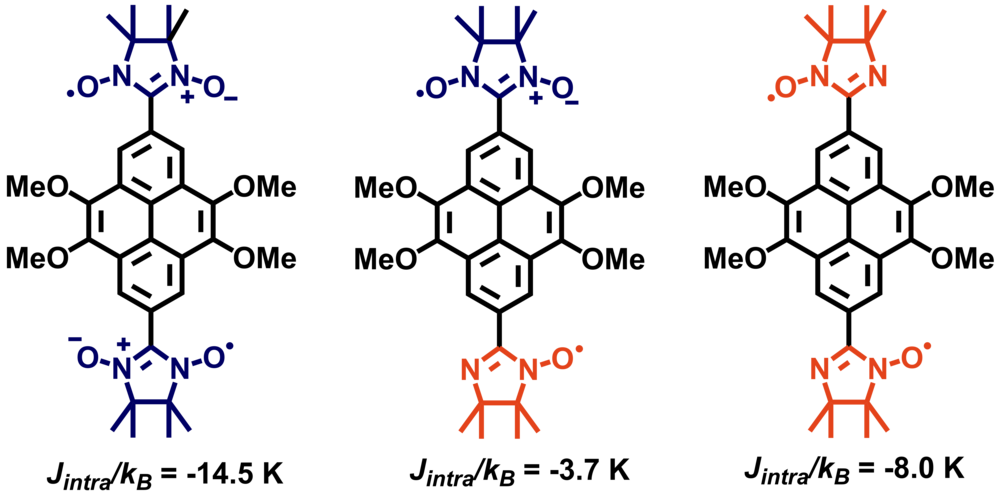
| 24 | [n]Helicene Diimides (n = 5, 6, and 7): Through-Bond versus Through-Space Conjugation M.Saal, F.; Zhang, F.; Holzapfel, M.; Stolte, M.; Michail, E.; Moos, M.; Schmiedel, A.; Krause, A.-M.; Lambert, C.; Würthner, F.; Ravat, P.* J. Am. Chem. Soc., 2020, 142, 21298–21303. DOI: 10.1021/jacs.0c11053 |

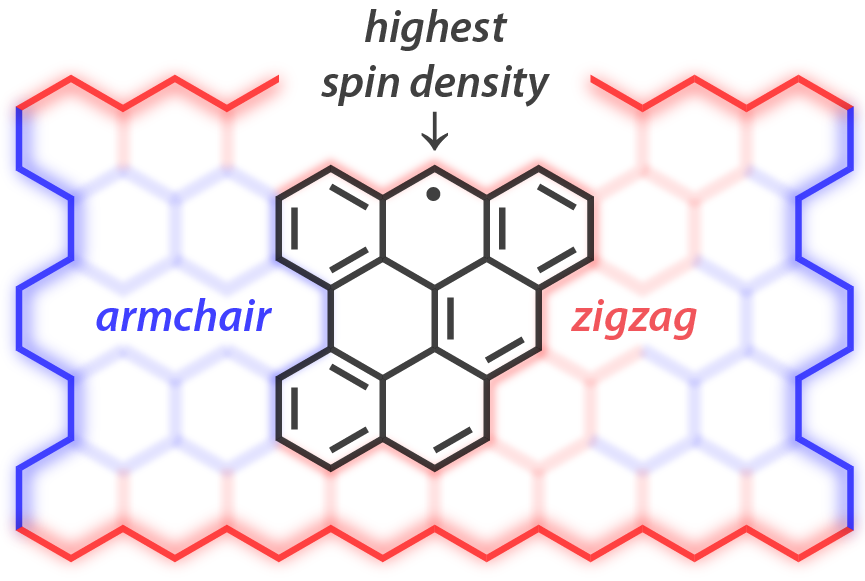
| 23 | Benzo[cd]triangulene: A Spin 1/2 Graphene Fragment Ravat, P.*; Blacque, O.; Juríček, M.* J. Org. Chem. 2020, 85, 92–100. |
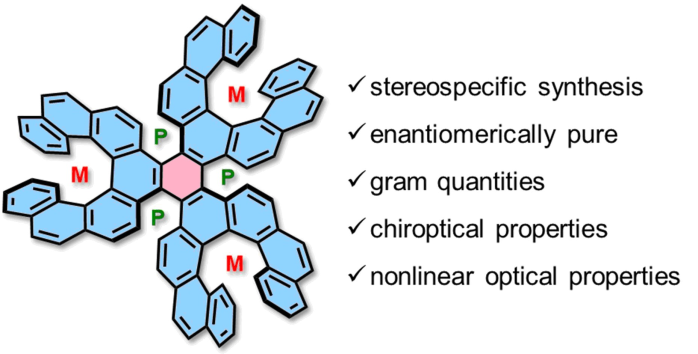
| 22 | Stereospecific Synthesis and Photophysical Properties of Propeller-Shaped C90H48 PAH Zhang, F.; Michail, E.; Saal, F.; Krause, A.-M.; Ravat, P.* Chem. Eur. J. 2019, 25, 16241–16245 . DOI: 10.1002/chem.201904962 |
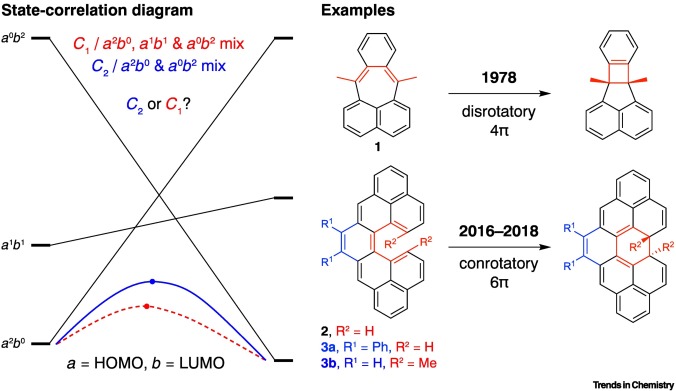
| 21 | Forbidden Electrocyclizations of Diradicaloids Šolomek, T.; Ravat, P.; Juríček, M. Trends in Chemistry 2019, 7, 705-706. DOI: 10.1016/j.trechm.2019.08.005 |
| 20 | Helicenes as Chiroptical Photoswitches Ravat, P.; Šolomek, T.; Juríček, M. ChemPhotoChem 2019, 3, 180-186. DOI: 10.1002/cptc.201800229 |
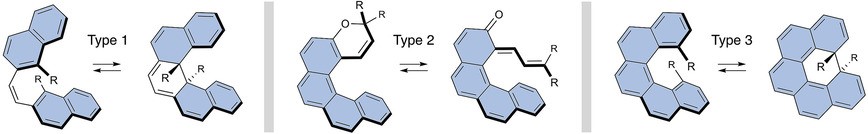
| 19 | Dimethylcethrene: A Chiroptical Diradicaloid Photoswitch Ravat, P.; Šolomek, T.; Häussinger, D.; Olivier, B.; Juríček, M. J. Am. Chem. Soc. 2018, 140, 10839-10847. DOI: 10.1021/jacs.8b05465 |
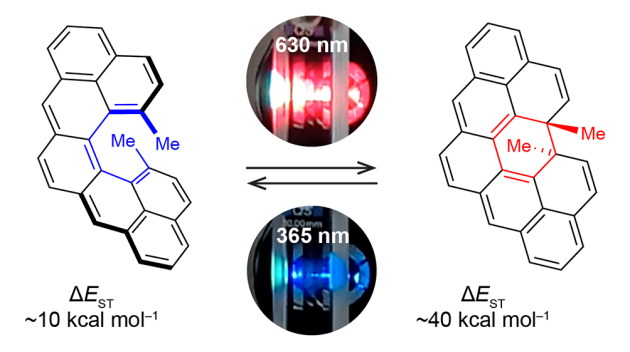
| 18 | Cethrene: The Chameleon of Woodward–Hoffmann Rules Šolomek, T.; Ravat, P.; Mou, Z.; Kertesz, M.; Juríček, M. J. Org. Chem. 2018, 83, 4769−4774. DOI: 10.1021/acs.joc.8b00656 |
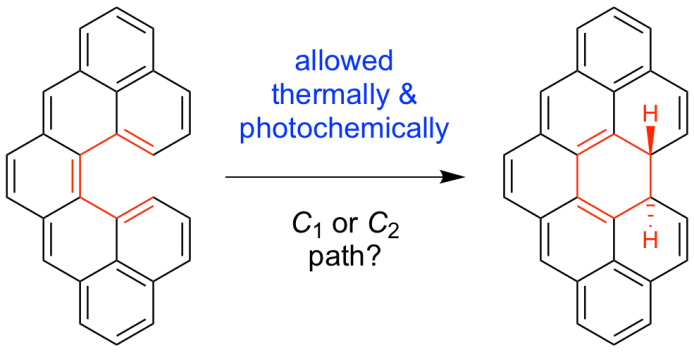
| 17 | Configurational Stability of [5]Helicenes Ravat, P.; Hinkelmann, R.; Steinebrunner, D.; Prescimone, A.; Bodoky, I.; Juríček, M. Org. Lett. 2017, 19, 3707−3710.DOI: 10.1021/acs.orglett.7b01461 |
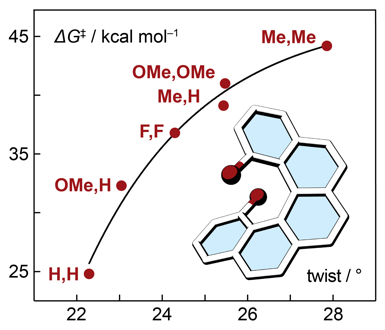
| 16 | Mixed Phenyl and Thiophene Oligomers for Bridging Nitronyl Nitroxides Kolanji, K.; Ravat, P.; Bogomyakov, A.; Ovcharenko, V.; Schollmeyer, D.; Baumgarten, M. J. Org. Chem. 2017, 82, 7764−7773. DOI: 10.1021/acs.joc.7b00435 |
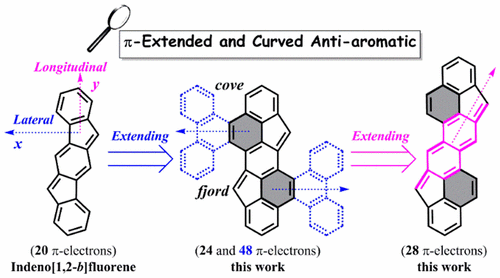
| 15 | π-Extended and Curved Anti-Aromatic Polycyclic Hydrocarbons Liu, J.; Ma, J.; Zhang, K.; Ravat, P.; Machata, P.; Avdoshenko, S. M.; Hennersdorf, F.; Komber, H.; Pisula, W.; Weigand, J. J.; Popov, A. A.; Berger, R.; Müllen, K.; Feng, X. J. Am. Chem. Soc. 2017, 139, 7513−7521. DOI: 10.1021/jacs.7b01619 Highlighted in Synfacts 2017, 13, 0931. |
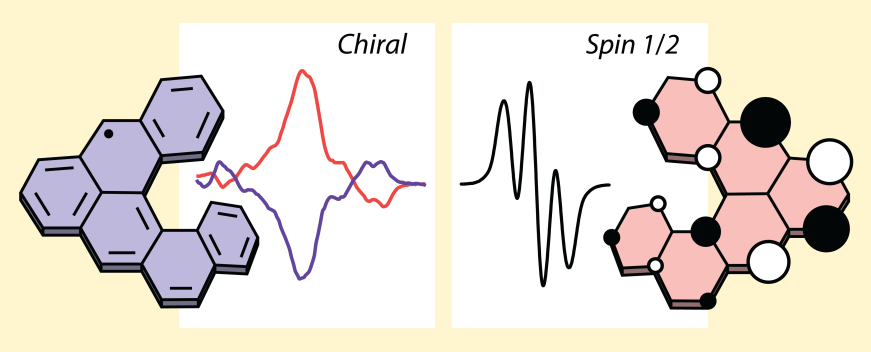
| 14 | Spin-Delocalization in a Helical Open-Shell Hydrocarbon Ravat, P.; Ribar, P.; Rickhaus, M.; Häussinger, D.; Neuburger, M.; Juríček, M. J. Org. Chem. 2016, 81, 12303−12317. DOI: 10.1021/acs.joc.6b02246 ACS Editors’ Choice. |
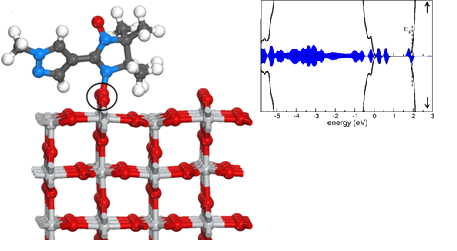
| 13 | Unraveling the Mark of Surface Defects on a Spinterface: the Nitronyl Nitroxide/TiO2(110) interface Kakavandi, R.; Calzolari, A.; Borozdina, Y. B.; Ravat, P.; Chassé, T.; Baumgarten, M.; Casu, M. B. Nano Res. 2016, 9, 3515−3527. DOI: 10.1007/s1227 |
| 12 | Biradicaloid with a Twist: Lowering the Singlet–Triplet Gap Ravat, P.; Šolomek, T.; Ribar P.; Juríček, M. Synlett 2016, 27, 1613−1617. Cover Article. DOI: 10.1055/s-0035-1561447 |
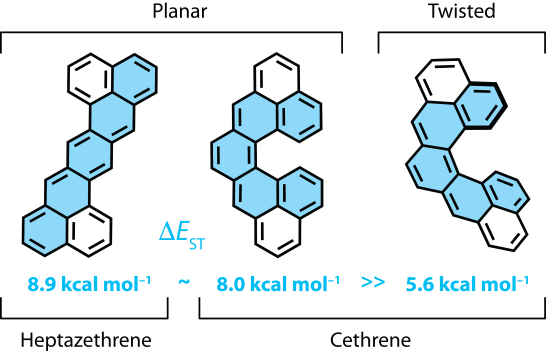
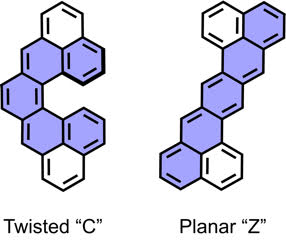
| 11 | Cethrene: A Helically Chiral Biradicaloid Isomer of Heptazethrene Ravat, P.; Šolomek, T.; Rickhaus, M.; Häussinger, D.; Neuburger, M.; Baumgarten, M.; Juríček, M. Angew. Chem. Int. Ed. 2016, 55, 1183−1187. DOI: 10.1002/anie.201507961 Highlighted in Chimia 2016, 70, 207 |
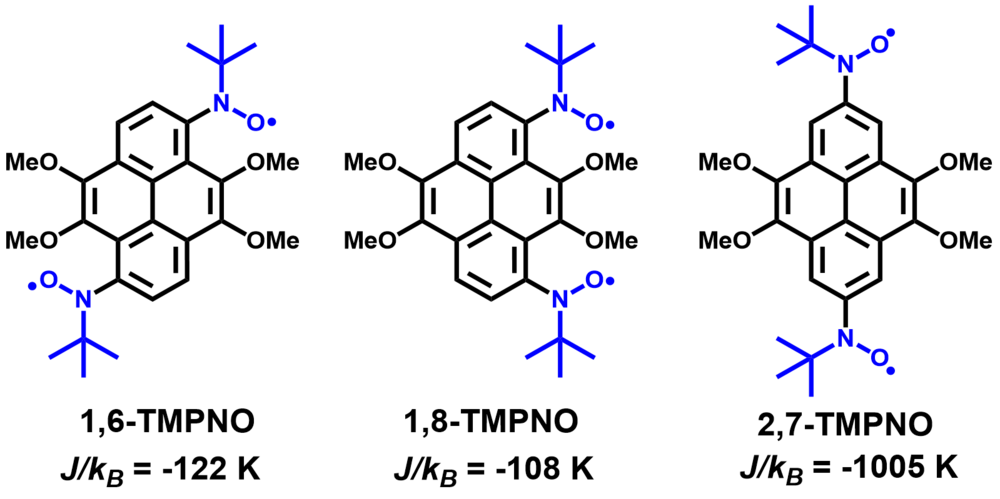
| 10 | Positional Isomers of Tetramethoxypyrene-Based Mono- and Biradicals Ravat, P.; Baumgarten, M. J. Phys. Chem. B 2015, 119, 13649−13655. DOI: 10.1021/acs.jpcb.5b03056 |
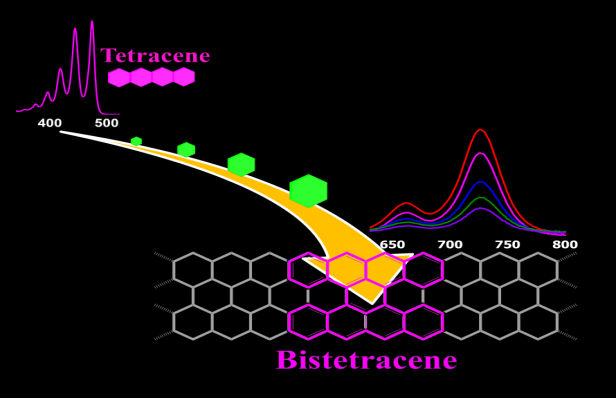
| 09 | Tetrabenzo[a,f,j,o]perylene: A Polycyclic Aromatic Hydrocarbon With An Open-Shell Singlet Biradical Ground State Liu, J.; Ravat, P.; Wegner, M.; Baumgarten, M; Feng, X.; Müllen, K. Angew. Chem. Int. Ed. 2015, 54, 12442−12446. DOI: 10.1002/anie.201502657 |
| 08 | Equivalence of Ethylene and Azo-Bridges in the Modular Design of Molecular Complexes: The Role of Weak Interactions Ravat, P.; SeethaLekshmi, S.; Biswas, S. N.; Nandy, P.; Varughese, S. Cryst. Growth Des. 2015, 15, 2389−2401. DOI: 10.1021/acs.cgd.5b00183 |
| 07 | Electronic Structure and Stability of Fluorophore-Nitroxide Radicals from Ultra High Vacuum to Air Exposure Kakavandi, R.; Ravat, P.; Savu, S.; Borozdina, Y.; Baumgarten, M.; Casu, B. ACS Appl. Mater. Interfaces 2015, 7, 1685−1692. DOI: 10.1021/am508854u |
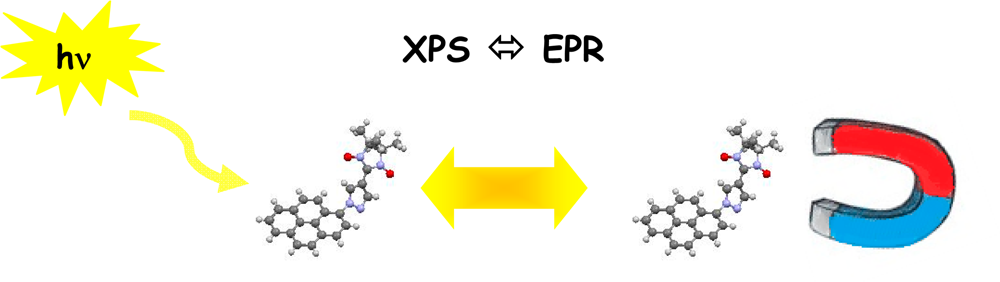
| 06 | Tschitschibabin type Biradicals: Benzenoid or Quinoid? Ravat, P.; Baumgarten, M. Phys. Chem. Chem. Phys. 2015, 17, 983−991. DOI: 10.1039/C4CP03522D |
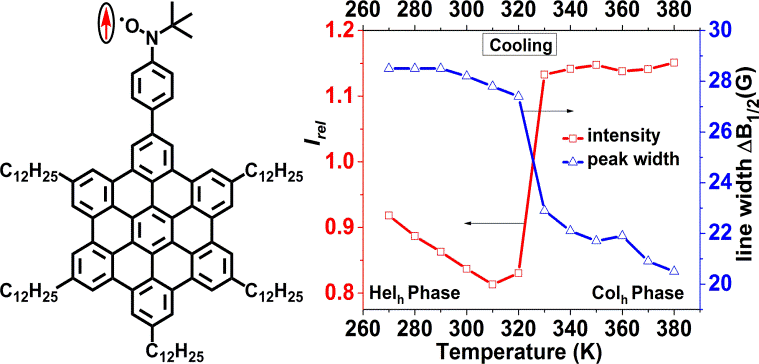
| 05 | Positive Magneto-LC Effect in Conjugated Spin-Bearing Hexabenzocoronene Ravat, P.; Marszalek, T.; Pisula, W.; Müllen, K.; Baumgarten, M. J. Am. Chem. Soc. 2014, 136, 12860−12863. DOI: 10.1021/ja507421x |
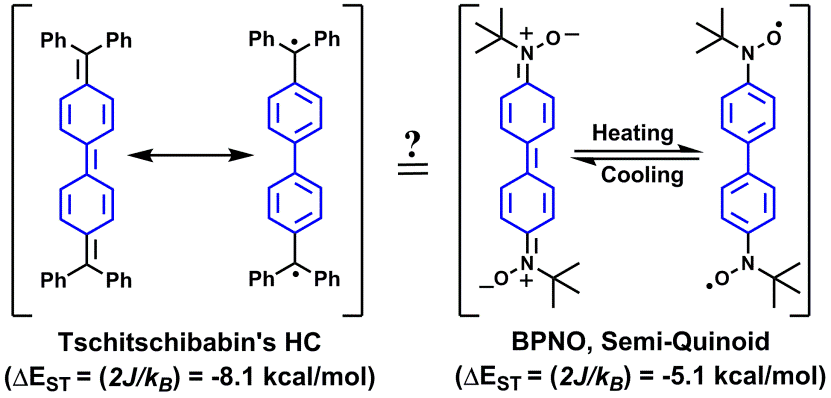
| 04 | Breaking the Semi-Quinoid Structure: Spin-Switching from Strongly Coupled Singlet to Polarized Triplet State Ravat, P.; Teki, Y.; Ito, Y.; Gorelik, E.; Baumgarten, M. Chem. Eur. J. 2014, 20, 12041−12045. DOI: 10.1002/chem.201403338 |
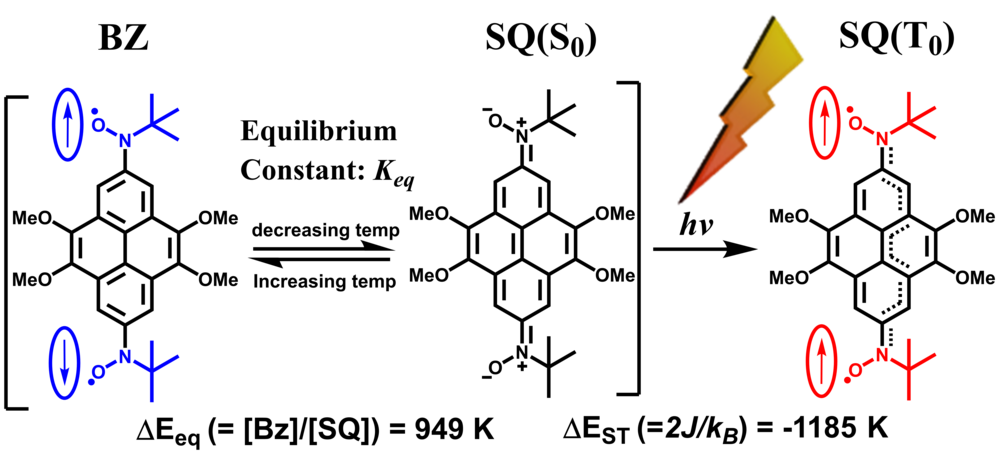
| 03 | Crystal Engineering of Tolane Bridged Nitronyl Nitroxide Biradicals: Candidates for Quantum Magnets Ravat, P.; Borozdina, Y.; Ito, Y.; Enkelmann, V.; Baumgarten, M. Cryst. Growth Des. 2014, 14, 5840−5846. DOI: 10.1021/cg5010787 |
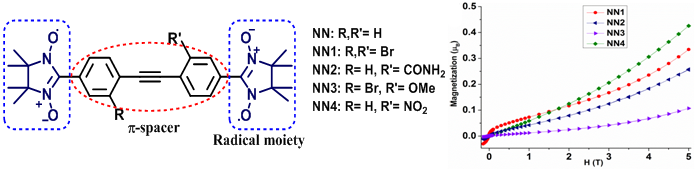
| 02 | Synthesis of Nitrogen-Doped ZigZag-Edge Peripheries: Dibenzo-9a-azaphenalene as Repeating Unit Berger, R.; Giannakopoulos, A.; Ravat, P.; Wagner, M.; Beljonne, D.; Feng, X.; Müllen, K. Angew. Chem. Int. Ed. 2014, 53, 10520−10524. DOI: 10.1002/anie.201403302 Highlighted in Synfacts 2014, 10, 1158. |
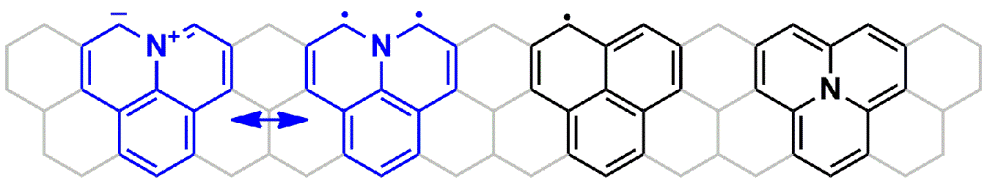
| 01 | Tetramethoxypyrene-Based Biradical Donors with Tunable Physical and Magnetic Properties Ravat, P.; Ito, Y.; Gorelik, E.; Enkelmann, V.; Baumgarten, M. Org. Lett. 2013, 15, 4280−4283. DOI: 10.1021/ol4015859 |