Research Topics
NMR Crystallography of Small Molecules
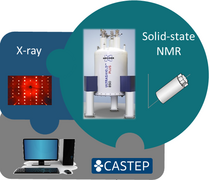
On the one hand, we use small molecule samples in the solid state to test the feasability and limits of specific NMR experiments for the subsequent investigation of drug-polymer formulations. On the other hand, we study the drug molecules separately using NMR Crystallography to gain insights into preferred interaction partners and the influence of different molecular arragements on NMR parameters. For that purpose, we also take a look at polymorphs, solvates and co-crystalline forms of the drug molecules as well as amorphous froms, thus complementing our understanding of the investigated compounds.
A structural model (often based on XRD data) is first geometry optimized before GIPAW (CASTEP) calculations yield a range of NMR parameters, which can be subsequently compared to experimental solid-state NMR data. This combination of experimental and calculated data helps us to detect polymorphic transformations and assign crowded spectra, in particular, for compounds with more than one molecule in the asymmetric crystallographic unit (Z' > 1). Furthermore, comparison between calculated NMR parameters for full crystal structures and separated individual molecules helps to predict changes in the experimental data to be expected upon variation of the coordination environment. Depending on the investigated compound, calculations are also done using the Gaussian software package.
A throrough understanding of the individual drugs is a prerequisite for the more complex analysis of the formulations.
Polymer-Drug-Formulations
Drugs can show low solubility in aqueous media, which can result in lower bioavailability and therefore might require higher drug dosing schemes and cause additional side-effects. Polymers can be used to increase the solubility of the drug, its circulation time and some are also known to reduce side effects. Furthermore, functional moieties on the particle surface enable enhancing the properties of such nanoparticles further.
The drug and the polymer can form diverse structures on the nm scale such as amorphous dispersions, micelles, vesicles and more. We want to gain a deeper insight into the behaviour of the molecules incorporated into polymers. In some cases, the rigid parts of such particles cannot be observed by NMR spectroscopy in solution. Therefore, we use solid-state NMR spectroscopy with moderate to fast MAS, where 2D correlations make it possible to observe molecular interactions between the drug and the polymer, which can result e.g. in a spatial model of a loaded micelle.[1] Investigation of the characteristics of these polymer-drug formulations is further done by PXRD, dissolution tests, etc. Combining every finding in understanding the intermolecular interactions, we investigate structure-property-relations and develop design for improved materials based on chemical modifications of the compounds.
The polymers we studied so far are predominantly synthesized by collaboration partners. We focussed on polymers based on poly-2-oxazolines[2,3] (working group of Prof. Luxenhofer, Helsinki, Finland) and poly(amino acids) (working group of Dr. Thornton, Leeds, UK). We are now expanding in this area to start making our own materials enabling us to study structure-property relations in more detail. On the drug-side, we use many different predominantly fluorine-containing compounds, as well as other poorly water-soluble as model substances.
Interested persons are recommended to have a look at the publications below or write an email to ann-christin.poeppler@uni-wuerzburg.de. Recently, Marvin published a paper about the investigation of paclitaxel – a drug for cancer treatment – by solid-state NMR methods, which he he also presented in the form of a snapshot video (link).[4] He also designed the inside front-cover using a photo of a Franconian cultural heritage.
[1] A.-C. Pöppler, M. M. Lübtow, J. Schlauersbach, J. Wiest, L. Meinel, R. Luxenhofer, Angew. Chem. Int. Ed. 2019, 58, 18540-18546.
[2] M. M. Lübtow, L. Hahn, M. S. Haider, R. Luxenhofer, J. Am. Chem. Soc. 2017, 139, 10980-10983.
[3] M. S. Haider, M. M. Lübtow, S. Endres, S. Forster, V. J. Flegler, B. Böttcher, V. Aseyev, A.-C. Pöppler, R. Luxenhofer, ACS Appl. Mater. Interfaces 2020, 12, 24531-24543.
[4] M. Grüne, R. Luxenhofer, D. Iuga, S. P. Brown, A. C. Pöppler, J. Mater. Chem. B 2020, 8, 6827-6836.
Behaviour (in biorelevant media/ with cells)
Drugs can be administered through different pathways. If the drug is supposed to be administered orally, the drug has to pass the stomach and gastrointestinal tract, where different challenges like harsh conditions (e.g. a very low pH), a multitude of other molecules and biological membrane obstacles are encountered before it can show its desired effects. The intestinal fluids, one such medium encountered on the journey of a drug formulation through the body, can be imitated by purchasable mixtures, termed biorelevant simulating media. The investigation by NMR spectroscopy in solution gives insights into the interactions as well as the effects of these simulated intestinal fluids on the drugs or drug-polymer-formulations and vice versa. Within a cooperation with the group of Prof. Groll, hydrogels for tissue engineering were also synthesized and investigated by NMR spectroscopy. This shows that also states in-between solid and solution can be thoroughly studied.
If you are interested to combine NMR spectroscopy with more biological environments, we can happily recommend you to take a look at the following articles or to write an e-mail to ann-christin.poeppler@uni-wuerzburg.de.
[1] S. Hanio, J. Schlauersbach, B. Lenz, I. Nischang, U. S. Schubert, S. Endres, A.-C. Pöppler, F. P. Brandl, T. M. Smit, K. Kolter, L. Meinel, accepted at Langmuir.
[2] J. Schlauersbach, S. Hanio, B. Lenz, S. Phani Babu Vemulapalli, C. Griesinger, A.-C. Pöppler, C. Harlacher, B. Galli, L. Meinel, J. Control. Release 2021, 330, 36-48.
[3] https://video.uni-wuerzburg.de/data-in-path/vod/upload_bac1268125ae959a7e46624d_12.14.18.mp4 Video to FaSSIF/FeSSIF