Research
Research
Electronic processes in functional π-systems are in focus of our group. We synthesise monomeric and polymeric π-conjugated compounds and investigate their electrochemical (cyclic voltammetry, spectroelectrochemistry in the UV/vis/NIR) and photophysical properties (steady-state absorption and emission spectroscopy, transient absorption spectroscopy in the fs- to µs time regime, fluorescence upconversion, magnetic-field dependent transient absorption measurements) concerning electron and energy transfer processes. A thorough understanding of these fundamental processes is a prerequisite for further development and optimisation of optoelectronic devices (e.g. OLED, OPV, OFET).
Low Band Gap Polymers
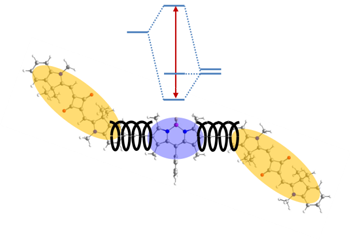
We synthesise squaraine dye homo- and copolymers with a low band-gap and spectrally broad absorption bands for light harvesting applications. The photophysical properties are explained using exciton coupling theory. Also low-molecular weight oligomers and conjugates with other dyes such as BODIPYs or pyrene are synthesised and investigated.
Energy Transfer Between Squaraine Polymer Sections: From Helix to Zigzag and All the Way Back
C. Lambert, F. Koch, S. F. Völker, A. Schmiedel, M. Holzapfel, A. Humeniuk, M. I. S. Röhr, R. Mitric, T. Brixner, J. Am. Chem. Soc. 2015, 137, 7851-7861.
Coupled Oscillators for Tuning Fluorescence Properties of Squaraine Dyes
C. Lambert, T. Scherpf, H. Ceymann, A. Schmiedel, M. Holzapfel, J. Am. Chem. Soc. 2015, 137, 3547-3557.
Organic Mixed-Valence Compounds
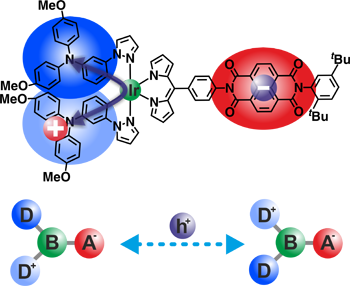
Our research on MV compounds provides the basis for a better understanding of electron or hole transfer processes between various redox centres such as triarylamines, perchlorinated triphenylmethyl radicals and triarylboranes. These redox centres are connected by saturated or conjugated bridges with different lengths and electronic properties. Besides (spectro)electrochemical and steady-state UV/Vis/NIR spectroscopy, dynamic EPR-spectroscopy helps us to analyse the optical and thermal electron transfer kinetics.
How fast is optically induced electron transfer in organic mixed valence systems?
C. Lambert, M. Moos, A. Schmiedel, M. Holzapfel, J. Schäfer, M. Keß, V. Engel, Phys. Chem. Chem. Phys. 2016, DOI: 10.1039/C6CP03053J.
B. Y. Mladenova, D. R. Kattnig, C. Kaiser, J. Schäfer, C. Lambert, G. Grampp, J. Phys. Chem. C 2015, 119, 8547-8553.
C. Lambert, R. Wagener, J. H. Klein, G. Grelaud, M. Moos, A. Schmiedel, M. Holzapfel, T. Bruhn, Chem. Commun. 2014, 50, 11350-11353.
Multidimensional Chromophores
A well-defined alignment of several chromophores or redox centres (e.g. as in hexaarylbenzenes) is a helpful prerequisite when studying electron or energy transfer processes because the orientation of the subunits determines many photophysical properties. Charge and energy transfer processes are investigated by steady-state and fs-time dependent fluorescence anisotropy spectroscopy.
H. Ceymann, A. Rosspeintner, M. H. Schreck, C. Mützel, A. Stoy, E. Vauthey, C. Lambert, Phys. Chem. Chem. Phys. 2016, 18, 16404-16413.
Energy redistribution dynamics in triarylamine-triarylborane containing hexaarylbenzenes
M. Steeger, M. Holzapfel, A. Schmiedel, C. Lambert, Phys. Chem. Chem. Phys. 2016, 18, 13403-13412.
Localised and delocalised excitons in star-like squaraine homo- and heterodimers
H. Ceymann, M. Balkenhohl, A. Schmiedel, M. Holzapfel, C. Lambert, Phys. Chem. Chem. Phys. 2016, 18, 2646-2657.
M. Steeger, S. Griesbeck, A. Schmiedel, M. Holzapfel, I. Krummenacher, H. Braunschweig, C. Lambert, Phys. Chem. Chem. Phys. 2015, 17, 11848-11867.
Donor-Acceptor Compounds
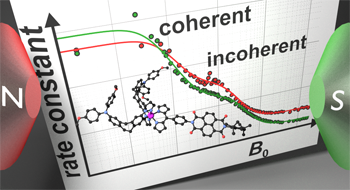
We investigate donor-acceptor compounds with redox centres being connected by organic bridges with different electronic properties. Further, we study donor-acceptor substituted transition metal complexes in which the transition metal complex acts as bridge and photosensitizer by time resolved and magnetic field dependent transient absorption spectroscopy. The goal is to achieve long-lived charge separated states and to use spin chemistry to influence the lifetime.
J. H. Klein, D. Schmidt, U. E. Steiner, C. Lambert, J. Am. Chem. Soc. 2015, 137, 11011-11021.
Photoinduced Electron Transfer Dynamics in Triarylamine-Naphthalene Diimide Cascades
F. Zieschang, M. H. Schreck, A. Schmiedel, M. Holzapfel, J. H. Klein, C. Walter, B. Engels, C. Lambert, J. Phys Chem C 2014, 118, 27698-27714.