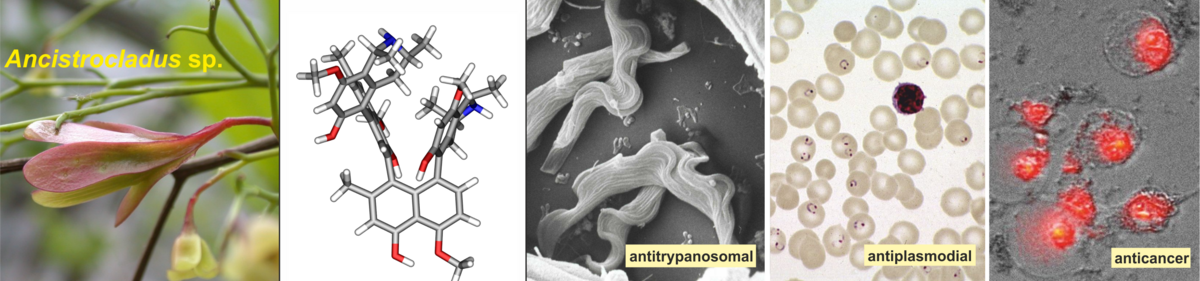
The research program of our group
Our group pursues natural products chemistry with structural, biosynthetic, and pharmacological facets. We approach this topic in a broad, highly interdisciplinary way, applying – and, in part, newly developing – novel efficient methods of analytical, synthetic, computational, and medicinal chemistry.
Our special interest is devoted to stereochemical questions of stereogenic centers (C- or N-based), axes (C,C or C,N, sp2-sp2 or sp2-sp3), of chiral ‘planes’ or helices, both analytically and synthetically, and we test and optimize their bioactivities. Starting from rewarding sources (mostly tropical plants), we search for novel antiparasitic, antileukemic, and anticancer natural products including the elucidation of their full absolute stereostructures. Using biomimetic or non-biomimetic strategies, we elaborate synthetic pathways to the most rewarding metabolites; as an example, we have developed the 'lactone method' for the atropo-selective construction of highly hindered axially chiral biaryl and hetero-biaryl systems of any desired configuration.
Naphthylisoquinoline Alkaloids: From the Plant to Promising Agents
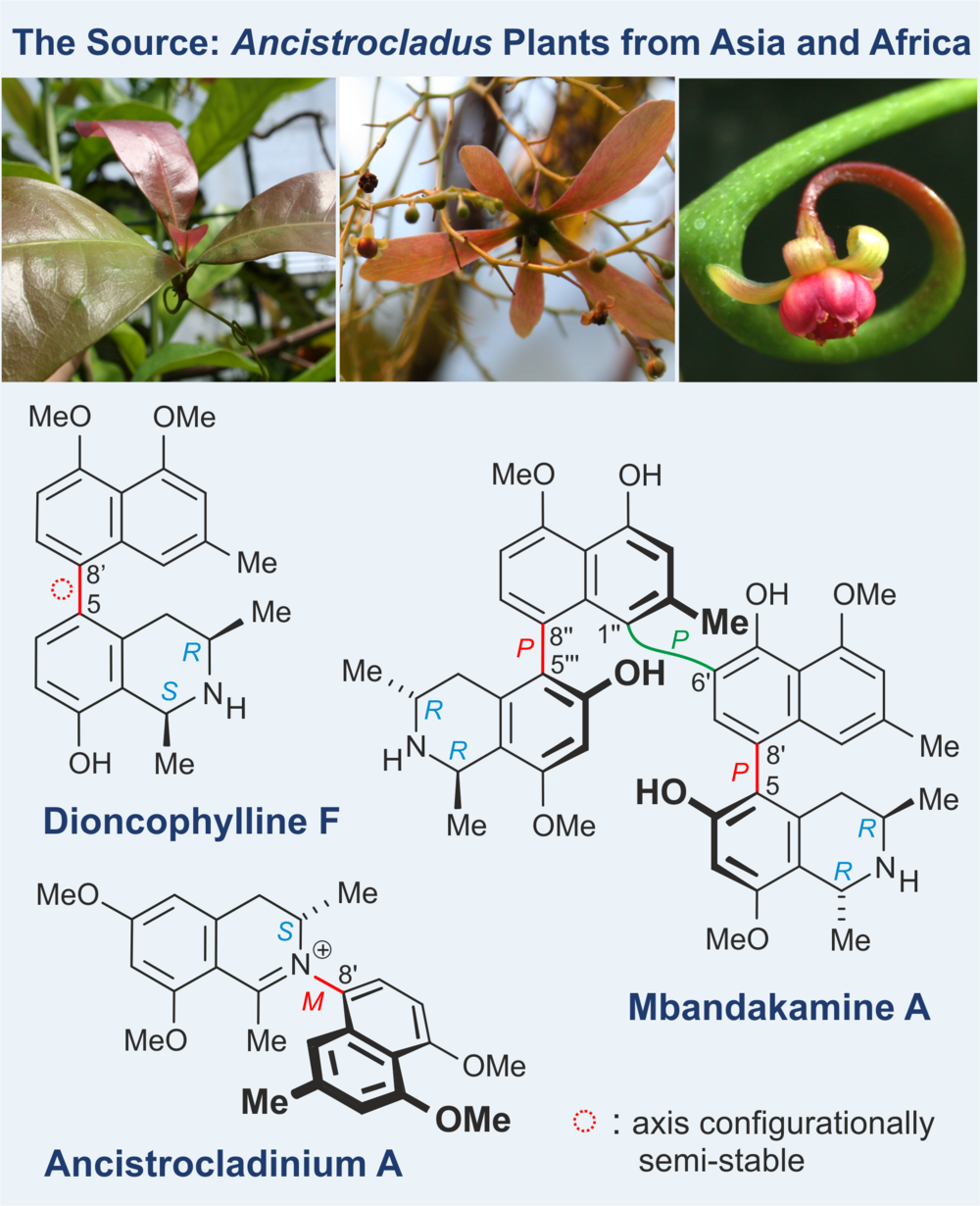
Naphthylisoquinoline alkaloids are structurally and biosynthetically extraordinary bioactive natural products possessing both, stereogenic centers and chiral axes. More than 200 representatives of this class of compounds have so far been identified, most of them by our group; they comprise three main structural subtypes:
- The by far highest number of alkaloids (ca. 220) are C,C-coupled monomers like e.g., dioncophylline F.
- A second, smaller subfamily (ca. 50 compounds) consists of structurally impressive dimeric alkaloids (likewise C,C-coupled) such as mbandakamine A with its three consecutive chiral biaryl axes.
- The smallest subclass (only 12 alkaloids), also showing a high degree of structural originality, are naphthylisoquinolines with an unprecedented stereogenic N-iminium aryl axis like e.g., ancistrocladinium A.
Depending on their individual structures, they display strong inhibitory effects against Plasmodium and Leishmania parasites, and they show promising antileukemic activities and potent cytotoxicities against PANC-1 human pancreatic cancer cells.
S. Fayez et al., RSC Adv. 2022, 12, 28916-28928; Naphthylisoindolinone alkaloids: the first ring-contracted naphthylisoquinolines, from the tropical liana Ancistrocladus abbreviatus, with cytotoxic activity DOI:10.1039/d2ra05758a
B.K. Lombe et al., J. Nat. Prod. 2021, 84, 1335-1344; Spirombandakamine A3 and Cyclombandakamines A8 and A9, Polycyclic Naphthylisoquinoline Dimers, with Antiprotozoal Activity, from a Congolese Ancistrocladus Plant DOI: 10.1021/acs.jnatprod.1c00063
S. Fayez et al., J. Nat. Prod. 2020, 83; 1139-1151; Ancistrosecolines A-F, Unprecedented seco-Naphthylisoquinoline Alkaloids from the Roots of Ancistrocladus abbreviatus, with Apoptosis-Inducing Potential against HeLa Cancer CellsDOI: 10.1021/acs.jnatprod.9b01168
Total Synthesis of Axially Chiral Natural Products
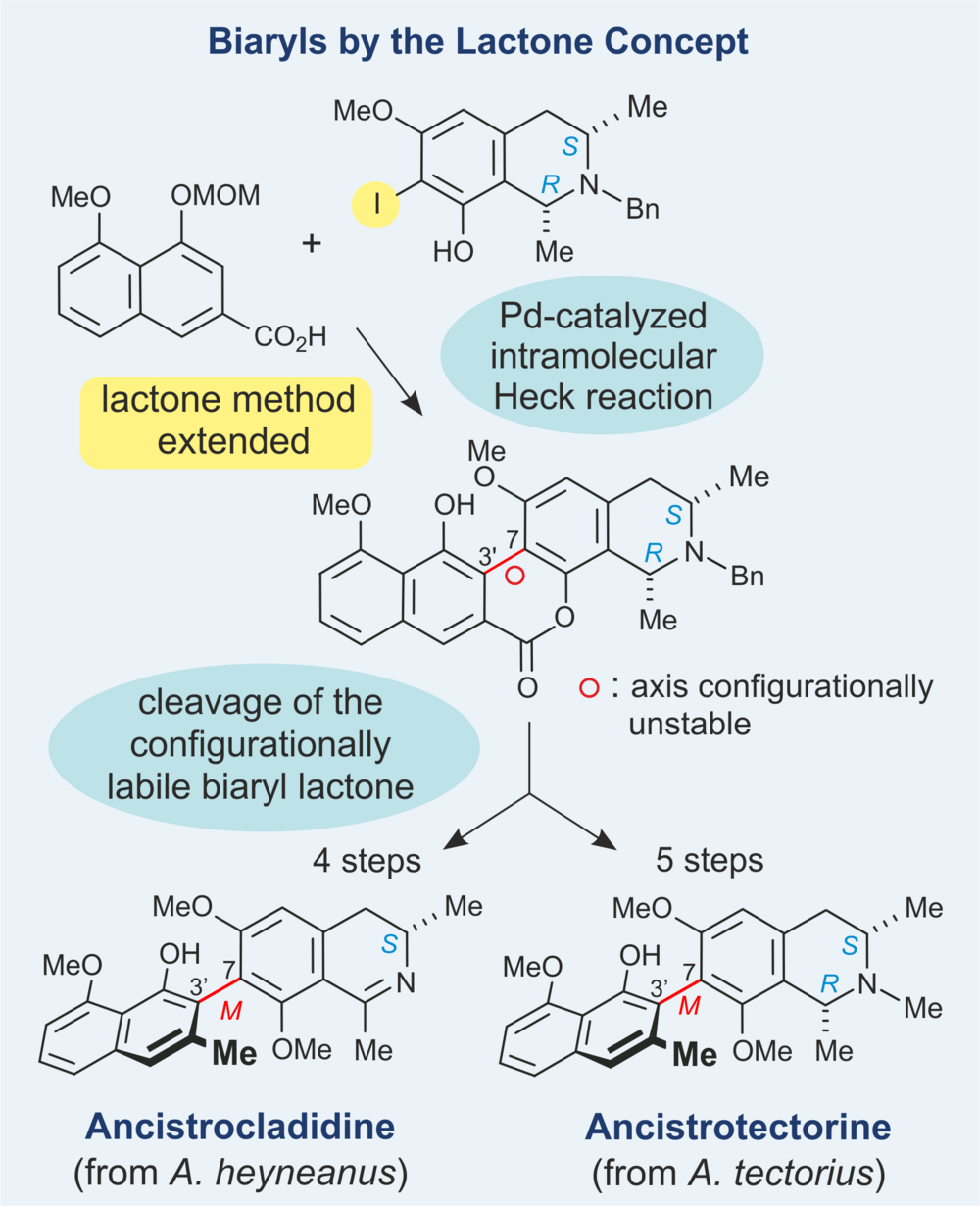
For the regio- and stereoselective formation of axially chiral biaryl systems, we have developed a fundamentally new approach, the 'lactone method'. It differs from other biaryl coupling methods by solving the two formal partial tasks of atropisomer-selective synthesis separately: the C-C bond formation and the asymmetric induction. The method has almost no restrictions concerning the substitution pattern, works even for substrates with high steric hindrance at the ortho-position next to the axis, and allows the optional, atropo-divergent preparation of either of the two atropisomers from the same biaryl lactone precursor.
We have extended the lactone method by activating the two molecular halves in an 'inverse-halogenated' form (and not as usually by introduction of the halogen function into the naphthoate building block). This was exemplified by the regio- and stereoselective total synthesis of the axially chiral 7,3'-coupled naphthylisoquinoline alkaloids ancistrocladidine and ancistrotectorine (see Scheme), i.e., by ester-type prefixation of the two molecular halves, followed by intramolecular coupling and atroposelective lactone ring cleavage.
Furthermore, our group aims at the total synthesis of axially chiral natural products by oxidative coupling reactions and at the convergent total synthesis of N,C-coupled naphthylisoquinoline alkaloids.
N. Tajuddeen et al., Acc. Chem. Res. 2022, 55, 2370-2383; The Stereoselective Total Synthesis of Axially Chiral Naphthylisoquinoline Alkaloids
DOI: 10.1021/acs.accounts.2c00432
Schies et al., ChemistrySelect 2018, 3, 940-945; Biomimetic Total Synthesis of Mbandakamine A and Further Antiplasmodial Naphthylisoquinoline Dimers
DOI: 10.1002/slct.201703160
Bringmann et al., Chem. Eur. J. 2016, 22; 9792-9796; First Atroposelective Total Synthesis of Enantiomerically Pure Ancistrocladidine and Ancistrotectorine
DOI: 10.1002/chem.20160
Assignment of Absolute Stereostructures by On-Line Methods
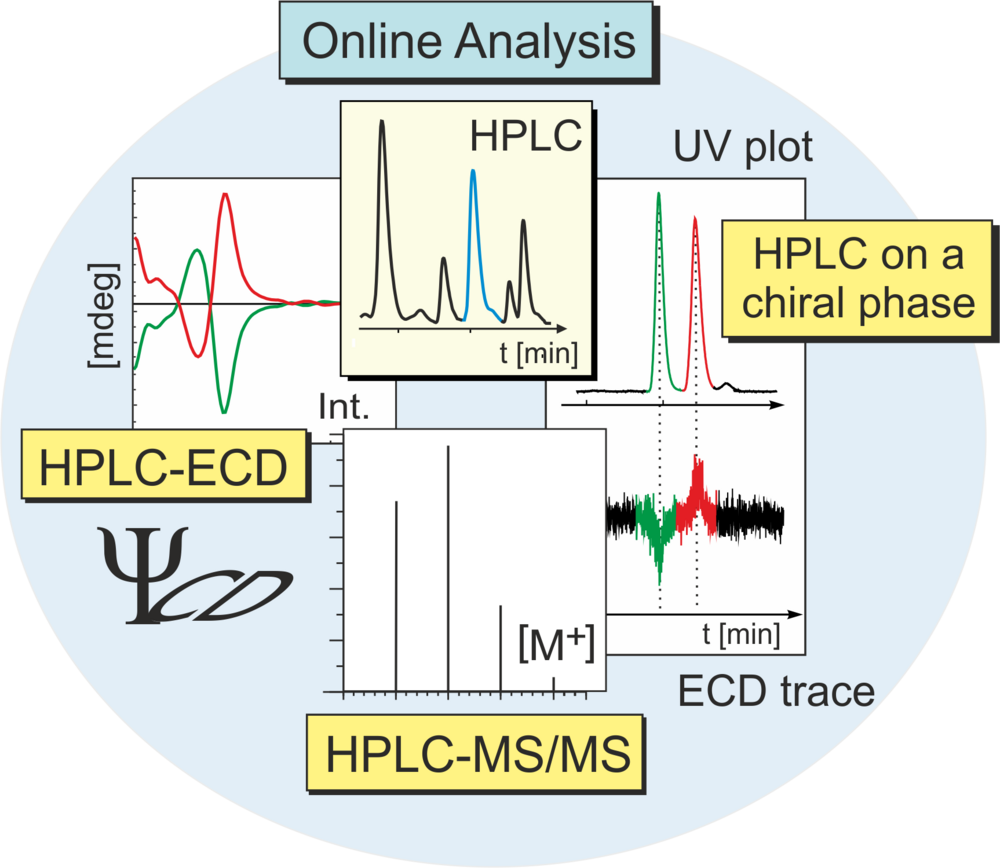
By the combination of UV, MS, NMR, and ECD methods with HPLC analysis – and in many cases by including quantum-chemical ECD calculations – we have successfully assigned the full absolute 3D structures of structurally most diverse natural and synthetic compounds with central, axial, helical, or planar chirality.
For the stereochemical analysis of chiral natural products, the HPLC-ECD hyphenation has become a most valuable tool. As an example, coupling of HPLC with ECD easily permits to directly distinguish between atropo-diastereomers or -enantiomers of naphthylisoquinoline alkaloids or between the stereoisomers of natural products occurring in the plant as scalemic mixtures. By HPLC on a chiral phase coupled with ECD, the absolute configuration of each enantio- or diastereomer, peak by peak trapped in the flow cell of the ECD spectropolarimeter, can be determined. For further selected examples of stereochemical assignments by LC-ECD hyphenation combined with quantum chemical ECD calculations: see refs. no. 3 and 4.
S. Fayez et al., Bioorg. Med. Chem. Lett. 2023, 129234; Dioncophyllidine E: The First Configurationally Semi-Stable 7,3'-Coupled Naphthylisoquinoline Alkaloid, from Ancistrocladus abbreviatus, with Antiausterity Activity against PANC-1 Human Pancreatic Cancer Cells
DOI: 10.1016/j.bmcl.2023.129234
D.T. Tshitenge et al., J. Nat. Prod. 2017, 80, 1604-1614; Gardenifolins A-H, Scalemic Neolignans from Gardenia ternifolia: Chiral Resolution, Configurational Assignment, and Cytotoxic Activities against the HeLa Cancer Cell Line
DOI: 10.1021/acs.jnatprod.7b00180
C. Schies et al., Chem. Commun. 2017, 53; 6121-6124; Metallocorroles as Inherently Chiral Chromophores: Resolution and Electronic Circular Dichroism Spectroscopy of a Tungsten Biscorrole
DOI: 10.1039/C7CC02027A
A. Gehrold et al., J. Org. Chem. 2016, 81, 1075-1088; Axial, Helical, and Planar Chirality in Directly Linked Basket-Handle Phorphyrin Arrays
DOI: 10.1021/acs.joc.5b02638