Just Published in Dalton Transactions
10/17/2017Catalyst-free room-temperature iClick reaction of molybdenum(II) and tungsten(II) azide complexes with electron-poor alkynes: structural preferences and kinetic studies
Authors: P. Schmid, M. Maier, H. Pfeiffer, L. Henry, A. Belz, A. Friedrich, F. Schönfeld, K. Edkins, U. Schatzschneider
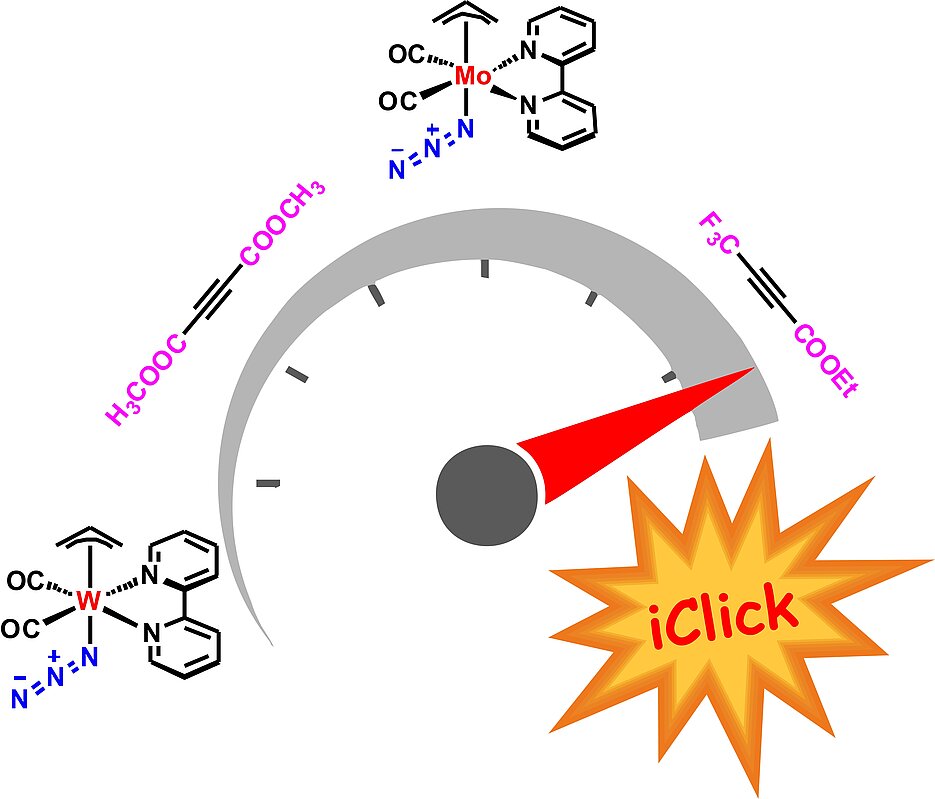
Two isostructural and isoelectronic group VI azide complexes of the general formula [M(η3-allyl)(N3)(bpy)(CO)2] with M = Mo, W and bpy = 2,2′-bipyridine were prepared and fully characterized, including X-ray structure analysis. Both reacted smoothly with electron-poor alkynes such as dimethyl acetylenedicarboxylate (DMAD) and 4,4,4-trifluoro-2-butynoic acid ethyl ester in a catalyst-free room-temperature iClick [3 + 2] cycloaddition reaction. Reaction with phenyl(trifluoromethyl)acetylene, on the other hand, did not lead to any product formation. X-ray structures of the four triazolate complexes isolated showed the monodentate ligand to be N2-coordinated in all cases, which requires a 1,2-shift of the nitrogen from the terminal azide to the triazolate cycloaddition product. On the other hand, a 19F NMR spectroscopic study of the reaction of the fluorinated alkyne with the tungsten azide complex at 27 °C allowed detection of the N1-coordinated intermediate. With this method, the second-order rate constant was determined as (7.3 ± 0.1) × 10−2 M−1 s−1, which compares favorably with that of first-generation compounds such as difluorocyclooctyne (DIFO) used in the strain-promoted azide–alkyne cycloaddition (SPAAC). In contrast, the reaction of the molybdenum analogue was too fast to be studied with NMR methods. Alternatively, solution IR studies revealed pseudo-first order rate constants of 0.4 to 6.5 × 10−3 s−1, which increased in the order of Mo > W and F3C–CC–COOEt > DMAD.