Bioactive Metal Complexes
Medicinal Inorganic Chemistry
Since the introduction of cisplatin as an important agent in the chemotherapy of cancer, there has been a steadily increasing interest in the exploration of metal-inherent properties for therapeutic applications in human medicine.
In fact, today, organometal and coordination compounds can be developed in a way totally in line with standard procedures of medicinal chemistry, and offer access to a significantly expanded chemical space due to a wide range of coordination geometries, facile variation of the coligand sphere, and tunable ligand exchange kinetics.
In addition to a traditional focus on anticancer agents, in recent years, more and more research has also been directed at the development of novel metal-based antimicrobial and antiviral agents, due to the urgent need to overcome antibiotics resistance to common agents and the threat of neglected tropical diseases (NTDs) as well as targeting of emerging viral infections.
Antiviral activity of (organo)metal complexes
Since late 2019, the COVID-19 pandemic has had a world-wide impact on public health and economy. In addition to vaccination, antiviral drugs might also contribute to fight the virus and provide a treatment option to persons already infected. As most of the SARS-CoV-2 viral proteins have now been isolated and characterized at the molecular level, the search is on for novel protein inhibitors. In a joint effort with many colleagues, we have started a research program that aims at the identification of metal complexes as inhibitors of the two key viral cysteine proteases 3CLpro and PLpro. In particular, we use a HPLC-based assay to study model protein degradation and organometal inhibitors of the relevant proteases.
- D. Graf, N. Farn, J. Klopf, M. Hojjati, U. Schatzschneider, Ferrocenoyl-substituted quinolinone and coumarin as organometallic inhibitors of SARS-CoV-2 3CLpro main protease, Metallomics 15, 23 (2023)
- M. Gil-Moles, S. Türck, U. Basu, A. Pettenuzzo, S. Bhattacharya, A. Rajan, X. Ma, R. Büssing, J. Wölker, H. Burmeister, H. Hoffmeister, P. Schneeberg, A. Prause, J. Kusi-Nimarko, S. Hassell-Hart, A. McGown, D. Guest, Y. Lin, A. Notaro; R. Vinck, J. Karges, K. Cariou, K. Peng, X. Qin, X. Wang, J. Skiba, L. Szczupak, K. Kowalski, U. Schatzschneider, C. Hemmert, H. Gornitzka, E.R. Milaeva, A.A. Nazarov, G. Gasser, J. Spencer, L. Ronconi, U. Kortz, J. Cinatl, D. Bojkova, I. Ott, Metallodrug profiling against SARS-CoV-2 target proteins identifies highly potent inhibitors of the spike/ACE2 interaction and the papain-like protease PLpro, Chem. Eur. J. 27, 17928–17940 (2021)
Antimicrobial activity of (organo)metal complexes
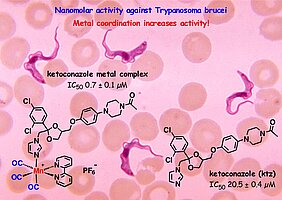
Increasing bacterial resistance to established antibiotics and very limited treatment options for neglected tropical diseases such as malaria and trypanosomiasis call for the development of new lead compounds. In that context, metal complexes have not been explored to their full potential so far, with the notable exception of ferroquine, an organometallic antimalarial drug candidate in advanced clinical trials. Thus, the Schatzschneider group has prepared a number of organometal complexes and identified a number of compounds with sub-micromolar biological activity in some cases:
- J.W. Betts, S. Cawthraw, J.A. Smyth, R.K. Poole, P. Roth, U. Schatzschneider, R.M. La Ragione, The manganese carbonyl complex [Mn(CO)3(tqa-κ3N)]Br: A novel antimicrobial agent with the potential to treat avian pathogenic Escherichia coli> (APEC) infections, Vet. Microbiol. 284, 109819 (2023)
- J.W. Betts, P. Roth, C.A. Pattrick, H.M. Southam, R. La Ragione, R.K. Poole, U. Schatzschneider, Antibacterial activity of Mn(I) and Re(I) tricarbonyl complexes conjugated to a bile acid carrier molecule, Metallomics 12, 1563-1575 (2020)
- A. Liakopoulos, R.M. La Ragione, C. Nagel, U. Schatzschneider, D. Rozen, J.W. Betts, Manganese complex [Mn(CO)3(tpa-κ3N)]Br restores antibiotic sensitivity to multidrug resistant Streptococcus pneumoniae, J. Glob. Antimicrob. Resist. 22, 594-597 (2020)
- J. Betts, C. Nagel, U. Schatzschneider, R. Poole, R.M. la Ragione, Antimicrobial activity of carbon monoxide-releasing molecule [Mn(CO)3(tpa-κ3N)]Br versus multidrug-resistant isolates of Avian Pathogenic Escherichia coli and its synergy with colistin, PLOS ONE 12, e0186359 (2017)
- N. Rana, H. Jesse, M. Tinajero-Trejo, J. Butler, M.L. von und zur Mühlen, C. Nagel, U. Schatzschneider, R.K. Poole, A manganese photosensitive tricarbonyl molecules [Mn(CO)3(tpa-k3N)]Br enhances antibiotic efficacy in a multi-drug-resistent Escherichia coli, Microbiology 163, 1477-1489 (2017)
- M. Tinajero-Trejo, N. Rana, T.W. Smith, C. Nagel, M.F. Hippler, U. Schatzschneider, R.K. Poole, Antimicrobial activity of the manganese photo-activated CO-releasing molecule [Mn(CO)3(tpa-κ3N)]+ against a multidrug-resistant Eschericha coli, Antioxid. Redox Signal. 24, 765-780 (2016)
- P.V. Simpson, C. Nagel, H. Bruhn, U. Schatzschneider, Antibacterial and antiparasitic activity of manganese(I) tricarbonyl complexes with ketoconazole, miconazole, and clotrimazole ligands, Organometallics 34, 3809-3815 (2015)
- C. Nagel, S. McLean, R.K. Poole, H. Braunschweig, T. Kramer, U. Schatzschneider, Introducing [Mn(CO)3(tpa-k3N)]+ as a novel photoactivatable CO-releasing molecule with well-defined iCORM intermediates - Synthesis, spectroscopy, and antibacterial activity, Dalton Trans. 43, 9986-9997 (2014)
- P.V. Simpson, C. Schmidt, I. Ott, H. Bruhn, U. Schatzschneider, Synthesis, cellular uptake and biological activity against pathogenic microorganisms and cancer cells of rhodium and iridium N-heterocyclic carbene metal complexes bearing charged substituents, Eur. J. Inorg. Chem. 5547-5554 (2013)
- L. Glans, W. Hu, C. de Kock, P.J. Smith, M. Haukka, H. Bruhn, U. Schatzschneider, E. Nordlander, Synthesis and biological activity of cymantrene and cyrhetrene 4-aminoquinoline conjugates against malaria, leishmaniasis, and trypanosomiasis, Dalton. Trans. 41, 6443-6450 (2012)
Anticancer activity of metal complexes and bioorganometallic compounds
In addition to cisplatin, a surprisingly wide range of other metal-coligand combinations also give rise to promising anticancer activity. Compounds evaluated in the group include rhodium(III) metalloinsertors, ruthenium(II) polypyridyl complexes, and molybdenum(II) allyl dicarbonyl compounds:
- V. Müller, P.V. Simpson, K. Peng, U. Basu, D. Moreth, C. Nagel, S. Türck, L. Oehninger, I. Ott, U. Schatzschneider, Taming the biological activity of Pd(II) and Pt(II) complexes with triazolato "protective" groups: 1H, 77Se NMR and X-ray crystallographic model studies with selenocysteine to elucidate differential thioredoxin reductase (TrxR) inhibition, Inorg. Chem. 62, 16203-16214 (2023)
- E. Schulz, V. Mawamba, M. Löhr, C. Hagemann, A. Friedrich, U. Schatzschneider, Structure-activity relations of Pd(II) and Pt(II) thiosemicarbazone complexes on different human glioblastoma cell lines, Z. Anorg. Allgem. Chem. 648, e202200073 (2022)
- H. Pfeiffer, M. Dragoun, A. Prokop, U. Schatzschneider, Biological activity of molybdenum(II) allyl dicarbonyl complexes with N-N coligands of variable aromatic surface area on adherent and non-adherent human cancer cells, Z. Anorg. Allgem. Chem. 639, 1568-1576 (2013)
- U. Schatzschneider, J. Niesel, I. Ott, R. Gust, H. Alborzinia, S. Wölfl, Cellular uptake, cytotoxicity, and metabolic profiling of ruthenium(II) polypyridyl complexes [Ru(bpy)2(N-N)]Cl2 with N-N = bpy, phen, dpq, dppz, and dppn, ChemMedChem 3, 1104-1109 (2008)
- U. Schatzschneider, J.K. Barton, Bifunctional rhodium intercalator conjugates as mismatch-directing DNA alkylating agents, J. Am. Chem. Soc. 126, 8630-8631 (2004)
Anticancer activity of organometal peptide and dendrimer conjugates
The cellular uptake and intracellular distribution of bioactive metal complexes can be modulated by conjugation to bio(macro)molecular carrier systems such as cell-penetrating peptides (CPPs) or dendrimers. We have extensively studied the anticancer activity of organometal compounds attached to such carrier peptides and obtained interesting results which raise the question of what is actually the carrier and what the cargo, the metal complex or the peptide?
- W. Hu, J. Hoyer, I. Neundorf, P. Govender, G.S. Smith, U. Schatzschneider, Synthesis of CpM(CO)3-DAB and PAMAM dendrimer conjugates and preliminary evaluation of their biological activity, Eur. J. Inorg. Chem. 1505-1510 (2015)
- W. Hu, K. Splith, I. Neundorf, K. Merz, U. Schatzschneider, Influence of the metal center and linker on the intracellular distribution and biological activity of organometal-peptide conjugates, J. Biol. Inorg. Chem. 17, 175-185 (2012)
- K. Splith, W. Hu, U. Schatzschneider, L.A. Onambele, A. Prokop, R. Gust, I. Ott, I. Neundorf, Protease-activatable organometal-peptide bioconjugates with enhanced cytotoxicity on cancer cells, Bioconjugate Chem. 21, 1288-1296 (2010)
- K. Splith, I. Neundorf, W. Hu, H.W. Peindy N'Dongo, V. Vasylyeva, K. Merz, U. Schatzschneider, Influence of the metal complex-to-peptide linker on the synthesis and properties of bioactive CpMn(CO)3 peptide conjugates, Dalton Trans. 39, 2536-2545 (2010)
- H.W. Peindy N'Dongo, I. Ott, R. Gust, U. Schatzschneider, Microwave-assisted solid-phase synthesis, cellular uptake, and cytotoxicity studies of cymantrene-peptide bioconjugates, J. Organomet. Chem. 694, 823-827 (2009)
- I. Neundorf, J. Hoyer, K. Splith, R. Rennert, H.W. Peindy N'Dongo, U. Schatzschneider, Cymantrene conjugation modulates the intracellular distribution and induces high cytotoxicity of a cell-penetrating peptide, Chem. Commun. 5604-5606 (2008)
- H.W. Peindy N'Dongo, I. Neundorf, K. Merz, U. Schatzschneider, Synthesis, characterization, and X-ray crystallography of cymantrene keto carboxylic acids for IR labelling of bioactive peptides on a solid support, J. Inorg. Biochem. 102, 2114-2119 (2008)
Bioimaging of metal complex distribution in living cells
The inherent spectroscopic signature of metal complexes, for example prominent M(C-O) bands in metal-carbonyl complexes, in a spectral window where the absorbance of cells and tissues is negligible, allowed us to image the biodistribution of such compounds with high spatial resolution in living human cells without fixation:
- K. Meister, J. Niesel, U. Schatzschneider, D. Schmidt, N. Metzler-Nolte, M. Havenith-Newen, Metal-carbonyl complexes as a new modality for label-free live cell imaging by Raman microspectroscopy, Angew. Chem. Int. Ed. 49, 3310-3312 (2010)