PUBLICATIONS
2024
49) Boron-containing helicenes as new generation of chiral materials: opportunities and challenges of leaving the flatland
A. Nowak-Król,* P. T. Geppert, K. R. Naveen
Chem. Sci. 2024, accepted (invited Perspective article)
48) Dual nature of thiophene harnessed in the design of near-infrared emitters via antiaromaticity relief
A. Nowak-Król*
Angew. Chem. Int. Ed. 2024, 63, e202317060.
DOI: 10.1002/anie.202317060
2023
47) Site-specific PEGylation of recombinant tissue-type plasminogen activator
K. Meiners, P. Hamm, M. Gutmann, J. Niedens, A. Nowak-Król, S. Pané, T. Lühmann*
Eur. J. Pharm. Biopharm. 2023, 192, 79-87.
DOI: 10.1016/j.ejpb.2023.09.017
46) Impact of truncation on optoelectronic properties of azaborole helicenes
J. Full, M. J. Wildervanck, C. Dillmann, S. P. Panchal, D. Volland, F. Full, K. Meerholz, A. Nowak-Król*
Chem. Eur. J. 2023, e202302808.
DOI: 10.1002/chem.202302808
45) Synthesis of a blue-emissive azaborathia[9]helicene by silicon-boron exchange from unusual atropisomeric teraryls
D. Volland, J. Niedens, P. T. Geppert, M. J. Wildervanck, F. Full, A. Nowak-Król*
Angew. Chem. Int. Ed. 2023, e202304291.
DOI: 10.1002/anie.202304291
Part of the Special Collecton "Rethinking Chemistry"
44) Amber light control of peptide secondary structure by a perfluoroaromatic azobenzene photoswitch
E. Cataldi, M. Raschig M. Gutmann, P. T. Geppert, M. Ruopp, M. Schock, H. Gerwe, R. Bertermann, L. Meinel, M. Finze, A. Nowak-Król, M. Decker, T. Lühmann*
ChemBioChem, 2023, 24, e202200570.
DOI: 10.1002/cbic.202200570.
2022
43) Electrophilic C–H Borylation of Aza[5]helicenes Leading to Bowl-Shaped Quasi-[7]Circulenes with Switchable Dynamics
X. Zhang,* F. Rauch, J. Niedens, R. B. da Silva, A. Friedrich, A. Nowak-Król,* Simon J. Garden,* T. B. Marder*
J. Am. Chem. Soc. 2022, 144, 22316–22324.
DOI: 10.1021/jacs.2c10865
highlighted in ChemistryViews
highlighted in Synfacts:
N→B-Bridged Quasi-[7]circulenes
D. Zhao, X. Bai
Synfacts 2023, 19, 0243.
DOI: 10.1055/s-0042-1753326
42) 55th Bürgenstock Conference under the banner of sustainability
A. Nowak-Król,* P. Dydio*
Angew. Chem. Int. Ed. 2022, 61, e202214722.
DOI: 10.1002/anie.202214722
Angew. Chem. 2022, 134, e202214722.
DOI: 10.1002/ange.202214722
Meeting Report
41) Synthesis and strong solvatochromism of push-pull thienylthiazole boron complexes
M. J. Wildervanck, R. Hecht, A. Nowak-Król*
Molecules 2022, 27, 5510.
DOI: 10.3390/molecules27175510.
Special Collection ‘New Boron Chemistry: Current Advances and Future Prospects’, invited
40) Synthesis of enantioenriched azaborole helicenes by chirality transfer from axially chiral biaryls
F. Full, M. J. Wildervanck, D. Volland, A. Nowak-Król*
Synlett 2023, 34, 477–482.
DOI: 10.1055/a-1914-1799.
SYNLETT Special Edition ‘Thieme Chemistry Journals Awardees 2022’
39) Enhanced Optical Properties of Azaborole Helicenes by Lateral and Helical Extension
F. Full, Q. Wölflick, K. Radacki, H. Braunschweig, A. Nowak-Król*
Chem. Eur. J. 2022, e202202280. (Hot Paper)
DOI: 10.1002/chem.202202280.
highlighted in Synfacts:
Azaborole Helicenes with Double Helical Axes
D. Zhao, X. Bai
Synfacts 2023, 19, 0143.
DOI: 10.1055/s-0042-1751818
38) Enhanced N-directed electrophilic C-H borylation generates BN- [5]- and [6]helicenes with improved photophysical properties
K. Yuan, D. Volland, S. Kirschner, M. Uzelac, G. S. Nichol, A. Nowak-Król,* M. J. Ingleson*
Chem. Sci. 2022, 13, 1136–1145.
DOI: 10.1039/D1SC06513K
Part of the Special Collection on "Emerging Frontiers in Aromaticity"
2021
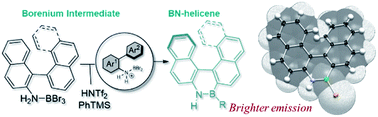
37) Modular synthesis of organoboron helically chiral compounds: cutouts from extended helices
J. Full, S. P. Panchal, J. Gӧtz, A.-M. Krause, A. Nowak-Król*
Angew. Chem. Int. Ed. 2021, 60, 4350–4357. (Hot Paper)
DOI: 10.1002/anie.202014138
Angew. Chem. 2021, 133, 4396–4403
DOI: 10.1002/ange.202014138
highlighted in ChemistryViews
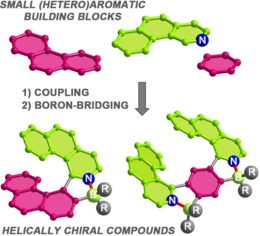
2020
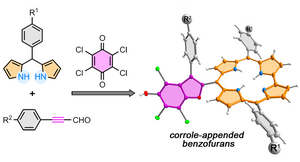
36) Access to corrole-appended persubstituted benzofurans by a multicomponent reaction: the dual role of p-chloranil
A. Nowak-Król,* B. Koszarna, M. Krzeszewski, T. D. Lohrey, J. Arnold,* D. T. Gryko*
Org. Lett. 2020, 22, 8139–8143.
DOI: 10.1021/acs.orglett.0c03133
2019
35) Ultrafast coherent exciton dynamics in size-controlled perylene bisimide aggregates S. Kang, C. Kaufmann, Y. Hong, W. Kim, A. Nowak-Król, F. Würthner, D. Kim Struct. Dyn. 2019, 6, 064501. DOI: 10.1063/1.5124148 34) Tetrahydroxy-perylene bisimide embedded in a zinc oxide thin film as an electron-transporting layer for high-performance non-fullerene organic solar cells X. Wen,# A. Nowak-Król,# O. Nagler, F. Kraus, N. Zhu, N. Zheng, M. Müller, D. Schmidt, Z. Xie, F. Würthner Angew. Chem. Int. Ed. 2019, 58, 13051-13055. DOI: 10.1002/anie.201907467 Angew. Chem. 2019, 131, 13185-13189 DOI:10.1002/ange.201907467 # equal contribution Press release 33) Low-melting porphyrins and their photophysical properties A. Nowak-Król, D. T. Gryko in Functional Organic Liquids; Ed. T. Nakanishi; Wiley-VCH, 2019 10.1002/9783527804948.ch2

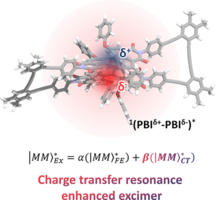
32) Solvent-modulated charge-transfer resonance enhancement in the excimer state of a bay-substituted perylene bisimide cyclophane W. Kim, A. Nowak-Król, Y. Hong, F. Schlosser, F. Würthner, D. Kim J. Phys. Chem. Lett. 2019, 10, 1919–1927. DOI: 10.1021/acs.jpclett.9b00357
31) Progress in the synthesis of perylene bisimide dyes A. Nowak-Król, F. Würthner Org. Chem. Front. 2019, 6, 1272-1318 (review). DOI: 10.1039/C8QO01368C
2018
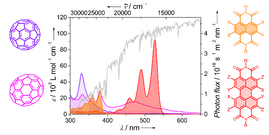
30) Naphthalene and perylene diimides – better alternatives to fullerenes for organic electronics? A. Nowak-Król, K. Shoyama, M. Stolte, F. Würthner Chem. Commun. 2018, 54, 13763-13772 (review). DOI: 10.1039/C8CC07640E
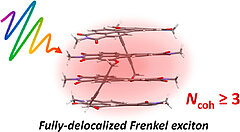
29) Ultrafast exciton delocalization, localization, and excimer formation dynamics in a highly defined perylene bisimide quadruple π‑stack C. Kaufmann, W. Kim, A. Nowak-Król, Y. Hong, D. Kim, F. Würthner J. Am. Chem. Soc. 2018, 140, 4253-4258. DOI: 10.1021/jacs.7b11571
2017
28) n-Channel organic semiconductors derived from air-stable four-coordinate boron complexes of substituted thienylthiazoles R. Hecht, J. Kade, D. Schmidt, A. Nowak-Król* Chem. Eur. J. 2017, 23, 11620-11628 (Hot Paper). DOI: 10.1002/chem.201701922
27) A crystalline π-stack containing five stereoisomers: insights into conformational isomorphism, chirality inversion, and disorder A. Nowak-Król, M. I. S. Röhr, D. Schmidt, F. Würthner Angew. Chem. Int. Ed. 2017, 56, 11774-11778. DOI: 10.1002/anie.201705445 Angew. Chem. 2017, 129, 11936-11940. DOI: 10.1002/ange.201705445
2016
26) Direct observation of excimer-mediated intramolecular electron transfer in a cofacially-stacked perylene bisimide pair
J. Sung, A. Nowak-Król, F. Schlosser, B. Fimmel, W. Kim, D. Kim, F. Würthner
J. Am. Chem. Soc. 2016, 138, 9029-9032.
DOI: 10.1021/jacs.6b04591
25) Stable, low-melting trans-A2B-corroles
A. Nowak-Król, E. Fourie, C. C. Joubert, D. Gryko, D. T. Gryko, J. C. Swarts
J. Porphyrins Phthalocyanines 2016, 20, 1244-1255.
DOI: 10.1142/S1088424616500942
24) Modulation of band gap and p- versus n-semiconductor character of ADA dyes by core and acceptor group variation
A. Nowak-Król, R. Wagener, F. Kraus, A. Mishra, P. Bäuerle, F. Würthner
Org. Chem. Front. 2016, 3, 545-555.
DOI: 10.1039/C6QO00046K
23) Tetramethoxy-bay-substituted perylene bisimides by copper-mediated cross-coupling
P. Leowanawat, A. Nowak-Król, F. Würthner
Org. Chem. Front. 2016, 3, 537-544 (front cover).
DOI: 10.1039/C6QO00047A
2015
22) Photoinduced electron transfer (PET) versus excimer formation in supramolecular p/n-heterojunctions of perylene bisimide dyes and implications for organic photovoltaics
A. Nowak-Król, B. Fimmel, M. Son, D. Kim, F. Würthner
Faraday Discuss. 2015, 185, 507-527.
DOI: 10.1039/C5FD00052A
21) Liquid-crystalline properties of trans-A2B2-porphyrins with extended π-electron systems
M. Salamończyk, D. Pociecha, A. Nowak-Król, D. Koszelewski, D. T. Gryko, E. Górecka
Chem.Eur.J. 2015, 21 ,7384–7388.
DOI: 10.1002/chem.201500296
20) An efficient synthesis of porphyrins with different meso substituents that avoids scrambling in aqueous media
A. Nowak-Król, R. Plamont, G. Canard, J. A. Edzang, D. T. Gryko, T. S. Balaban
Chem. Eur. J. 2015, 21, 1488-1498 (inside cover).
DOI: 10.1002/chem.201403677
19) The 1H, 13C, 15N, and 19F NMR chemical shifts assignments in 5,10,15-tris(penta-fluorophenyl)tetra-15N corrole at 191 K
W. Bocian, P. Paluch, A. Nowak-Król, D. T. Gryko, M. Potrzebowski, J. Śniechowska, J. Sitkowski, E. Bednarek, L. Kozerski
Magn. Res. Chem. 2015, 53, 167-171.
DOI: 10.1002/mrc.4145
2014
18) Soluble meso-tetrakis(arylethynyl)porphyrins - synthesis and optical properties
A. Nowak-Król, Ł. G. Łukasiewicz, J. E. Haley, M. Drobizhev, A. Rebane, T. M. Cooper, D. T. Gryko
J. Porphyrins Phthalocyanines 2014, 18, 998-1013.
DOI: 10.1142/S1088424614500904
17) Two-photon absorption in butadiyne-linked porphyrin dimers: torsional and substituent effects
J. D. Wilkinson, G. Wicks, A. Nowak-Król, Ł. G. Łukasiewicz, C. J. Wilson, M. Drobizhev, A. Rebane, D. T. Gryko, H. L. Anderson
J. Mat. Chem. C 2014, 2, 6802-6809.
DOI: 10.1039/C4TC01120A
16) Insights into the tautomerism in meso-substituted corroles: a variable-temperature 1H, 13C, 15N, and 19F NMR spectroscopy study
S. Szymański, P. Paluch, D. T. Gryko, A. Nowak-Król, W. Bocian, J. Sitkowski, B. Koszarna, J. Śniechowska, M. J. Potrzebowski, L. Kozerski
Chem. Eur. J. 2014, 20, 1720-1730.
DOI: 10.1002/chem.201303406
2013
15) Oxidative aromatic coupling of meso-arylamino-porphyrins
A. Nowak-Król, D. T. Gryko
Org. Lett. 2013, 15, 5618-5621.
DOI: 10.1021/ol4022035
14) Study of intermolecular interactions in the corrole matrix by solid-state NMR under 100 kHz MAS and theoretical calculations
T. Kobayashi, K. Mao, P. Paluch, A. Nowak-Król, J. Sniechowska, Y. Nishiyama, D. T. Gryko, M. J. Potrzebowski, M. Pruski
Angew. Chem. Int. Ed. 2013, 52, 14108-14111.
DOI: 10.1002/anie.201305475
Angew. Chem. 2013,125, 14358-14361.
DOI: 10.1002/ange.201305475
13) All-optical corrole-based oxygen sensor
N. Czechowski, A. Nowak-Król, D. T. Gryko, S. Maćkowski
Phys. Scr. 2013, T157, 014009.
DOI: 10.1088/0031-8949/2013/T157/014009
12) Strong two-photon absorption enhancement in a unique bis-porphyrin bearing a diketopyrrolopyrrole unit
A. Nowak-Król, M. Grzybowski, J. Romiszewski, M. Drobizhev, G. Wicks, M. Chotkowski, A. Rebane, E. Górecka, D. T. Gryko
Chem. Commun. 2013, 49, 8368-8370.
10.1039/C3CC44728F
11) Synthesis and linear and nonlinear optical properties of low-melting π-extended porphyrins
D. Koszelewski, A. Nowak-Król, M. Drobizhev, C. J. Wilson, J. E. Haley, T. M. Cooper, J. Romiszewski, E. Górecka, H. L. Anderson, A. Rebane, D. T. Gryko
J. Mater. Chem. C 2013, 1, 2044-2053.
DOI: 10.1039/C3TC00594A
2012
10) Fluorescence microscopy of corrole-single silver nanowire hybrid nanostructures
N. Czechowski, M. Olejnik, A. Nowak-Król, D. Piątkowski, W. Heiss, D.T. Gryko, S. Mackowski
Acta Phys. Pol. A 2012, 122, 333-336.
DOI: 10.12693/APhysPolA.122.333
9) Amplified two-photon absorption in trans-A2B2-porphyrins bearing nitrophenylethynyl substituents
A. Nowak-Król, C. J. Wilson, M. Drobizhev, D. V. Kondratuk, A. Rebane, H. L. Anderson, D. T. Gryko
ChemPhysChem 2012, 13, 3966-3972 (Editor’s selection).
DOI: 10.1002/cphc.201200507
8) Selective cycloaddition of tetracyanoethene (TCNE) and 7,7,8,8-tetracyano-p-quinodimethane (TCNQ) to afford meso-substituted phenylethynyl porphyrin
D. Koszelewski, A. Nowak-Król, D. T. Gryko
Chem. Asian J. 2012, 7, 1887-1894.
DOI: 10.1002/asia.201200179
2011
7) Synthesis of trans-A2B2-porphyrins bearing phenylethynyl substituents
A. Nowak-Król, B. Koszarna, S. Y. Yoo, J. Chromiński, M. K. Węcławski, C.-H. Lee, D. T. Gryko
J. Org. Chem. 2011, 76, 2627-2634.
DOI: 10.1021/jo1025578
2010
6) Mass spectrometry studies on meso-substituted corroles and their photochemical decomposition products
P. Świder, A. Nowak-Król, R. Voloshchuk, J. P. Lewtak, D. T. Gryko, W. Danikiewicz
J. Mass Spectrom. 2010, 45, 1443-1451.
DOI: 10.1002/jms.1860
5) meso-Alkylidene (m-benzi)pentaphyrin: a modified pentaphyrin bearing exocyclic double bonds at meso-positions
S.-D. Jeong, A. Nowak-Król, Y. Kim, S.-J. Kim, D. T. Gryko, C.-H. Lee
Chem. Commun. 2010, 8737-8739.
DOI: 10.1039/C0CC03263H
4) Synthesis and adrenolytic activity of new propanolamines
G. Groszek, A. Bajek, A. Bis, A. Nowak-Król, M. Bednarski, A. Siwek, B. Filipek
Molecules 2010, 15, 3887-3904.
DOI: 10.3390/molecules15063887
3) Meso-substituted liquid porphyrins
A. Nowak-Król, D. Gryko, D. T. Gryko
Chem. Asian J. 2010, 5, 904-909 (cover article).
DOI: 10.1002/asia.200900693
2009
2) Synthesis and adrenolytic activity of 1-(1H-indol-4-yloxy)-3-(2-(2-methoxy-phenoxy)ethylamino)propan-2-ol analogs and its enantiomers. Part 2
G. Groszek, A. Nowak-Król, T. Wdowik, D. Świerczyński, M. Bednarski, M. Otto, M. Walczak, B. Filipek
Eur. J. Med. Chem. 2009, 44, 5103-5111.
DOI: 10.1016/j.ejmech.2009.07.012
2008
1) Straightforward transformation of pentafluorobenzaldehyde into 4-aryloxy-2,3,5,6-tetrafluorobenzaldehydes
D. T. Gryko, D. Wyrostek, A. Nowak-Król, K. Abramczyk, M. K. Rogacki
Synthesis 2008, 4028-4032.
DOI: 10.1055/s-0028-1083246
PATENTS
1) Nowe blokery optyczne oparte na bis-porfirynach o rozszerzonym chromoforze (New blockers based on bis-porphyrins with extended chromophores)
D. T. Gryko, A. Nowak-Król
Polish granted patent PL 223427 B1, 2016.