Silicon-Containing Reagents in Synthesis
TMOP as a Protecting Group (TMOP = 2,4,6-Timethoxyphenyl)
Advantages of the 2,4,6-Trimethoxyphenyl Protecting Group:
- Selective introduction with commercially available reagents
- Easy to characterize by NMR spectroscopy
- Is stable under basic, neutral, and oxidative conditions
- Highly selective cleavage under very mild conditions (0 °C) with simultaneous introduction of a reactive Si–Cl bond
- The byproduct of the cleavage (1,3,5-trimethoxybenzene) is pH-neutral and relatively inert and can be largely removed by crystallization from unpolar solvents
F. Popp, J. B. Nätscher, J. O. Daiss, C. Burschka, R. Tacke, Organometallics 2007, 26, 6014–6028. more ...
TMOP as a Protecting Group (TMOP = 2,4,6-Timethoxyphenyl)
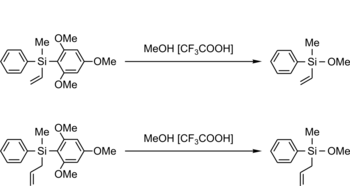
F. Popp, J. B. Nätscher, J. O. Daiss, C. Burschka, R. Tacke, Organometallics 2007, 26, 6014–6028. more ...
Selective Cleavage of Methoxyphenyl Protecting Groups
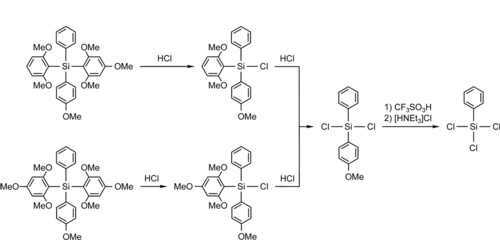
F. Popp, J. B. Nätscher, J. O. Daiss, C. Burschka, R. Tacke, Organometallics 2007, 26, 6014–6028. more ....

