Fast Energy Transport between Unlike Partners
10/04/2016Chemists from the University of Würzburg have combined different dye molecules in aggregates and thereby observed surprising properties. Their discovery may help to use sunlight more efficiently for the generation of energy.
Plants use accumulations of dye molecules, so-called light-harvesting complexes, to capture sunlight and to convert water (H2O) and carbon dioxide (CO2) to energy-rich organic compounds and oxygen (O2) through photosynthesis. For this, they have to transport the energy gained by light absorption over numerous dye molecules to the photosynthetic reaction centers. Individual dye units, so-called chromophores, are kept in close proximity by the surrounding protein shell to ensure efficient energy transfer.
Two Balls Linked by a Spring
The optical absorption properties of the light-harvesting complexes differ significantly from the ones of the individual dye molecules. This is due to the so-called exciton coupling between the dye molecules. The coupling can be visualized by two balls hanging on threads linked by a spring. If one ball is moved from its position of rest, the second ball will be also influenced. On molecular level the displacement of the ball corresponds to the excitation of a molecule through light absorption.
This phenomenon is well understood for systems comprising like dye molecules (homo-aggregates) while little is known about coupling between different chromophores. New insights currently come from the group of Professor Frank Würthner, chairholder of Organische Chemie II at the University of Würzburg and director of the Center for Nanosystems Chemistry. The multidisciplinary journal Nature Communications reports on this in its online version.
Quadruple Stacks of Dye Molecules
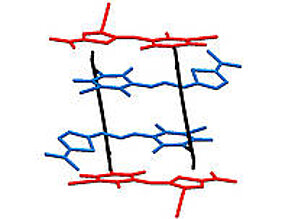
The investigation of coupling between dye molecules requires aggregates with distinct orientation of the dye units. The coworkers of Professor Würthner succeeded in preparing appropriate aggregates in the form of stacks comprising four chromophores. For this they used merocyanine dyes which form well-defined aggregates due to their strong dipolar character. “By the chemical linkage of two equal chromophores via a naphthalene unit we could obtain a molecule that dimerizes in solution forming stacks of four equal chromophores”, explains David Bialas, PhD student in the group of Frank Würthner and author of the publication.
Afterwards, the scientist went one step further: they linked two different merocyanine chromophores exhibiting different absorption properties to obtain hetero-aggregates in form of quadruple stacks.
“The structure of the dye stacks in solution could be investigated with the help of nuclear magnetic resonance spectroscopy”, says Eva Kirchner, who is also a PhD student in the group and was involved in the project. An unambiguous proof for the existence of the quadruple stacks was obtained by X-ray structural analysis. For this, the team had to grow suitable crystals, which is a challenging task for dye aggregates.
Unexpected Absorption Properties
The observations during the spectroscopic studies of the absorption properties were unexpected. “The results indicated exciton coupling between the dye molecules not only for the homo-aggregate but also for the hetero-aggregate”, explains Bialas. Quantum mechanical calculations confirmed a strong exciton coupling between the different types of chromophores in the hetero-aggregate. “This contradicts the common belief that strong coupling is only possible between the same types of chromophores”, says the scientist.
Fast Energy Transfer
Exciton coupling not only influences the absorption properties of dye aggregates but is also an indication for fast energy transfer between the molecules. This could be used by scientists in the future to harvest sunlight more efficiently since the usage of different dye molecules allows to cover a broader absorption of the solar spectrum. Thus, more energy may be gained which can be converted to current or chemical energy.
Contact
Prof. Dr. Frank Würthner, Institute of Organic Chemistry of the University of Würzburg
Phone: +49 931 31-85340, wuerthner@chemie.uni-wuerzburg.de