Ring expansion reactions of NHCs and related molecules
Since the isolation of bottleable N-heterocyclic carbenes in 1991 by Arduengo et al. the chemistry of NHCs and related molecules has grown rapidly and numerous applications of NHCs in main group and transition metal chemistry, as well as catalysis, have been reported. Usually these NHCs provide a robust environment and are often regarded as “innocent” ligands. Within the coordination sphere of transition metals, however, constructive and destructive decomposition pathways of NHC ligands are well established, which mainly involve C-H, C-N, C-C, and N-H bond activation steps of the nitrogen alkyl, aryl or hydrogen substituents. We presented earlier – at that time one of the rare examples – the observation of a ring expansion reaction of NHCs in the presence of hydrosilanes (D. Schmidt, J. H. J. Berthel, S. Pietsch, U. Radius, Angew. Chem. Int. Ed. 2012, 51, 8881–8885). The insertion of (formally) silylene moieties into the C-N bond of N-heterocyclic carbenes leads to ring expansion and formation of diazasilinanes. These reactions are feasible using primary, secondary and tertiary silanes Ph4-nSiHn and a variety of NHCs, i.e. saturated and unsaturated NHCs with N-alkyl and N-aryl substituents. This ring expansion should be of general interest for the different areas of NHC-based chemistry of main group elements and transition metals, as well as for catalysis, especially if the NHC ligand is labile and a certain set of substrates is used for catalysis. In other words, within this project we are looking for ways to destroy our NHCs in a sensitive way and test the limitations of these “innocent” co-ligands.
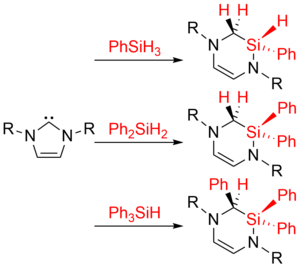
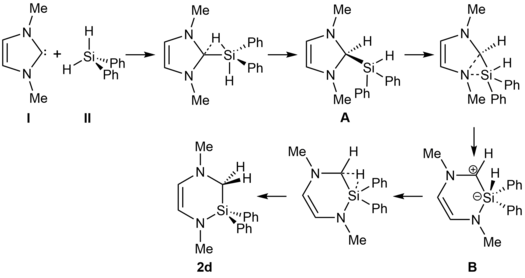
We provided in the manuscript a tentative mechanism for the reaction, which was confirmed by DFT and ab initio calculations performed by other groups afterwards.
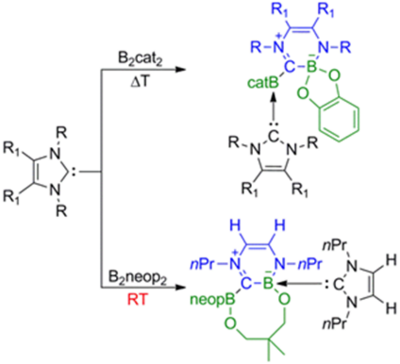
In collaboration with Todd Marder and Mike Ingleson reported then that NHC ring expansion might be a facile process, especially if diborane(4) compounds are used (D. Pietsch, U.Paul, I. A. Cade, M. J. Ingleson, U. Radius, T. B. Marder, Chem. Eur. J. 2015, 21, 9018–9021). The reaction of NHCs and bis(catecholato)diboron (B2cat2) leads to neutral mono- and bis-NHC adducts of bis(catecholato)diboron (B2cat2) depending on the nature of the diborane and the carbene. Some of these bis-NHC adducts undergo thermally induced rearrangement, forming a 6-membered -B–C=N–C=C–N-heterocyclic ring via C–N bond cleavage and ring expansion of the NHC, whereas the mono-NHC adduct is stable. Bis(neopentylglycolato)diboron (B2neop2), for example, is much more reactive than B2cat2 giving a ring expanded product even at room temperature !! These findings clearly show that ring expansion of NHCs can be a very facile process with significant implications for their use in catalysis. Thus, our current example at room temperature suggests that this non-innocent behavior of NHCs could play an important role in catalyst deactivation both in metal-catalyzed and metal free processes utilizing NHCs as ligands or as catalysts themselves.