Thioether & Sulfoxide
Thioethers & Sulfoxides
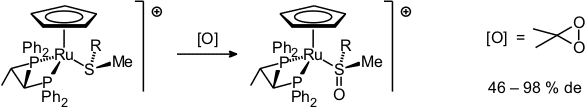
Organometallic thioethers as well as thiolate complexes can be readily and selectively oxidized with dimethyldioxirane [57]. The same oxidant can be employed to convert coordinated thioethers to the corresponding sulfoxides. This reaction when carried out on chiral thioether complexes gives the sulfoxides in good to excellent diastereoselectivity [69, 77, 85].
We have later used this reaction in a transition metal-mediated synthesis of (R)-sulforaphane, a natural product occurring in broccoli, cauliflower, and related species [78]. (R)-Sulforaphane is known for its ability to protect mammals from the adverse effects of chemical carcinogens.
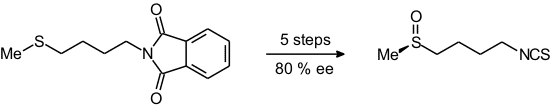
A further extension of this methodology involves the selective oxidation of allyl thioethers to (sulfinylmethyl)epoxides. Here, the ruthenium complex acts as a protective group which suppresses the double oxidation of the thioether function [79, 85, 98].