A Choise of Recent Publications 2015 - 2017
Recent Publications of Tackes' Research Group 2015-2017
| 21. | |
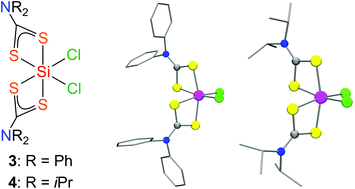
| 20. | J. A. Baus, R. Tacke, Neutral six-xoordinate bis(dithiocarbamato)-silicon(IV) complexes with an SiCl2S4 skeleton, Dalton Trans., in print (2017.) http://dx.doi.org/10.1002/ejic.201600982more ... AbstractTreatment of SiCl4 with lithium dithiocarbamates of the formula type Li[R2NCS2] (R = Ph, iPr) in a molar ratio of 1 |
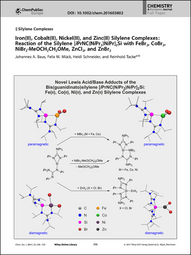
| 19. | J. A. Baus, F. M. Mück, H. Schneider, R. Tacke, Iron(II), cobalt(II), and zinc(II) silylene complexes: reaction of the silylene [i(PrNC(NiPr2)NiPr2]Si with FeBr2, CoBr2, NiBe2 • MeOCH2CH2OMe, ZnCl2, and ZnBr2, Chem. Eur. J. 23, 296-303 (2017). http://dx.doi.org/10.1002/ejic.201600982more ...
Abstract Donor-Stabilized Silylenes The chemistry of donor-stabilized silylenes has developed into one of the most actively studied research areas in molecular silicon chemistry. In their Full Paper on page 296 ff., R. Tacke et al report on the synthesis and characterization of novel transition-metal silylene complexes of FeBr2, CoBr2, NiBr2, ZnBr2, and ZnCl2 with the bis(guanidinato)silicon(II) complex [iPrNC(NiPr2)NiPr]2Si. These compounds represent very rare examples of carbonyl-free silylene complexes of 3d transition metals in the formal oxidation state +2. |
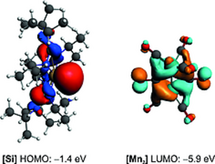
| 18. | J. A. Baus, J. Poater, F. M. Bickelhaupt, R. Tacke, Silylene-induced reduction of [Mn2(CO)10]: formation of a five-coordinate silicon(IV) complex with an O-bound [(OC)4Mn=Mn)CO)4]2- ligand, Eur. J. Inorg. Chem. 186-191 (2017). http://dx.doi.org/10.1002/ejic.201600982more ...
Abstract: Based on a new silylene-induced reduction of [Mn2(CO)10], a five-coordinate silicon(IV) complex was synthesized, which is formally built up by an anionic [(OC)4Mn=Mn(CO)4]2– and a cationic {[iPrNC(NiPr2)NiPr]2Si}2+ fragment, which are connected by a nearly linear Si–O–C moiety. The Si–O and the C–O bond of this Si–O–C moiety display partial double-bond character. |
| 17. | J. A. Baus, N. Laskowski, R. Tacke, A Silacyclopropene with a six-coordinate silicon atom and an SiN4C2 skeleton: synthesis and structural characterization, Eur. J. Inorg. Chem. 5182-5184 (2016). more ... |
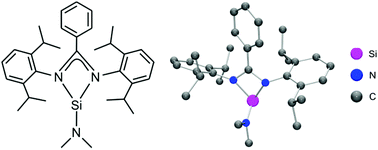
| 16. | J. A. Baus, F. M. Mück, R. Bertermann, R. Tacke, Homoleptic two-coordinate 14-electron palladium and platinum complexes with two bis(guanidinato)silylene ligands, Eur. J. Inorg. Chem. 4867-4871 (2016). more ... |
| 15. | F. M. Mück, J. A. Baus, C. Burschka, R. Bertermann, R. Tacke, Lewis acid/base reactions of the bis(amidinato)silylene [iPrNC(Ph)NiPr]2Si with ElPh3 (El = B, Al), Organometallics 35, 2583-2588 (2016). more ... |
| 14. | F. M. Mück, B. Förster, J. A. Baus, M. Nutz, C. Burschka, R. Bertermann, R. Tacke, Four-, five-, and six-coordinate silicon(IV) complexes: reactivity of the donor-stabilized silylenes [iPrNC(Ph)NiPr]2Si and [iPrNC(NiPr2)NiPr]2Si towards Me3SiN3 and PhSCH2N3, Eur. J. Inorg. Chem. 3246-3252 (2016). more ... |
| 13. | F. M. Mück, J. A. Baus, M. Nutz, C. Burschka, R. Bertermann, R. Tacke, SO2 activation by the bis(guanidinato)silylene [iPrNC(NiPr2)NiPr]2Si: formation of neutral six-coordinate silicon(IV) complexes with a chelating sulfito or dithionito ligand, Eur. J. Inorg. Chem. 3240-3245 (2016). more ... http://dx.doi.org/10.1002/ejic.201600402 |
| 12. | F. M. Mück, J. A. Baus, C. Burschka, R. Tacke, Cationic five-coordinate bis(guanidinato)silicon(IV) complexes with SiN4El skeletons (El = S, Se): "Heterolytic activation" of S-S and Se-Se bonds, Chem. Eur. J. 22, 5830-5834 (2016). more ... |
| 11. | F. M. Mück, J. A. Baus, A. Ulmer, C. Burschka, R. Tacke, Reactivity of the donor-stabilized guanidinatosilylene [ArNC(NMe2)NAr]Si[N(SiMe3)2] (Ar = 2,6-Diisopropylphenyl), Eur. J. Inorg. Chem. 1660-1670 (2016). more ... |
| 10. | J. Ehbets, S. Lorenzen, C. Mahler, R. Bertermann, A. Berkefeld, J. Poater, E. Fritz-Langhals, R. Weidner, F. M. Bickelhaupt, R. Tacke, Synthesis and hydrolysis of alkoxy(aminoalkyl)diorganylsilanes of the formula type R2(RO)Si(CH2)nNH2 (R = Alkyl, n = 1-3): A systematic experimental and computational study, Eur. J. Inorg. Chem. 1641-1659 (2016). more ... |
| 9. | M. Geyer, J. A. Baus, O. Fjellström, E. Wellner, L. Gustafsson, R. Tacke, Synthesis and pharmacological properties of silicon-sontaining GPR81 and GPR109A agonists, ChemMedChem 10, 2063-2070 (2015). more ...
|
| 8. | F. M. Mück, J. A. Baus, M. Nutz, C. Burschka, J. Poater, F. M. Bickelhaupt, R. Tacke, Reactivity of the donor-stabilized silylenes [iPrNC(Ph)NiPr]2Si and [iPrNC(NiPr2)NiPr]2Si: Activation of CO2 and CS2, Chem. Eur. J. 21, 16665-16672 (2015). more ... |
| 7. | K. Samedov, R. West, P. W. Percival, J.-C. Brodovitch, L. Chandrasena, M. Mozafari, R. Tacke, K. Junold, C. Kobelt, P. O. Samuel, R. Azakahar, K. C. Mondal, H. W. Roesky, M. Driess, W. Wang, Free radicals of N-donor-stabilized silicon(II) compounds probed by Muon spin spectroscopy, Organometallics 34, 3532-3537 (2015). more ... |
| 6. | F. M. Mück, D. Kloß, J. A. Baus, C. Burschka, R. Bertermann, J. Poater, C. Fonseca Guerra, F. M. Bickelhaupt, R. Tacke, Stable four-coordinate guanidinatosilicon(IV) complexes with SiN3El skeletons (El = S, Se, Te) and Si=El double bonds, Chem. Eur. J. 21, 14011-14021(2015). more ... |
| 5. | R. Tacke, C. Kobelt, J. A. Baus, R. Bertermann, C. Burschka, Synthesis, structure and reactivity of a donor-stabilised silylene with a bulky bidentate benzamidinato ligand, Dalton Trans. 44, 1459-14974 (2015). more ... The novel donor-stabilised silylene 5 was prepared in a four-step synthesis, starting from bis(2,6-diisopropylphenyl)carbodiimide (Dipp–NCN–Dipp), and its reactivity was studied in a series of oxidative addition reactions and a nucleophilic substitution reaction. The three-coordinate silicon(II) complex 5 contains the bulky bidentate amidinato ligand Dipp–NC(Ph)N–Dipp− and a dimethylamido ligand. Treatment of 5 with N2O afforded the dinuclear five-coordinate silicon(IV) complex 6 (SiO2N3 skeletons), and the reaction with S8 yielded the dinuclear four-coordinate silicon(IV) complex 7 (SiS2N2 skeletons). Treatment of 5 with Se and Te afforded the respective four-coordinate silicon(IV) complexes 8 (SiSeN3 skeleton) and 9 (SiTeN3 skeleton), which contain an SiSe and SiTe double bond, respectively. The reaction of 5 with the silyl azide Me3SiN3 yielded the four-coordinate silicon(IV) complex 10 (SiN4 skeleton) with an SiN double bond, whereas the reaction with the alkyl azide PhSCH2N3 gave the four-coordinate silicon(IV) complex 11 (SiSN3 skeleton), the first silicon(IV) complex with an unsubstituted methyleneamido ligand. The reaction of 5 with [Fe(CO)5] afforded the four-coordinate silicon(II) complex 12 (SiFeN3 skeleton) with an Si–Fe bond. Compounds 5–12 (and the precursors 14 and 15 (five-coordinate silicon(IV) complexes with an SiCl3N2 and SiCl2N3 skeleton, respectively) in the synthesis of 5) were characterised by elemental analyses, crystal structure analyses and multinuclear NMR spectroscopic studies in the solid state and in solution. |
| 4. | P. Luger, B. Dittrich, R. Tacke, Invariom based electron density studies on the C/Si analogues haloperidol/sila-haloperidol and venlafaxine/sila-venlafaxine, Org. Biomol. Chem. 13, 9093-9106 (2015). more ...
|
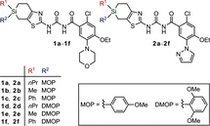
| 3. | M. Geyer, O. Karlsson, J. A. Baus, E. Wellner, R. Tacke, Si- and C-functional organosilicon building blocks for synthesis based on 4-silacyclohexan-1-ones containing the silicon protecting groups MOP (4-methoxyphenyl), DMOP (2,6-dimethoxyphenyl), or TMOP (2,4,6-trimethoxyphenyl), J. Org. Chem. 80, 5804-5811 (2015). more ... |
| 2. | M. Geyer, E. Wellner, U. Jurva, S. Saloman, D. Amstrong, R. Tacke, Can silicon make an excellent drug even better? An in vitro and in vivo head-to-head comparison between loperamide and its silicon analogue sila-loperamide, ChemMedChem 10, 911-924 (2015). more ...  Carbon/silicon switch: Sila-loperamide (1b), a silicon analogue of the opioid receptor agonist loperamide (1a), was synthesized. A head-to-head comparison of the in vitro pharmacodynamics and pharmacokinetics and the in vivo pharmacokinetics of 1a and 1b revealed that the C/Si switch strategy is a smart choice to modulate potency, efficacy, stability, and metabolic fate. |
| 1. | F. M. Mück, A. Ulmer, J. A. Baus, C. Burschka, R. Tacke, Thermally stable four-coordinate silicon(IV) complexes with an Si=N double bond and an SiN3X skeleton (X = O, S, Se, Te), Eur. J. Inorg. Chem. 1860-1864 (2015). more ... |

 Dalton Trans. 46, 13628-13659 (2017), Issue 40, 2017
Dalton Trans. 46, 13628-13659 (2017), Issue 40, 2017