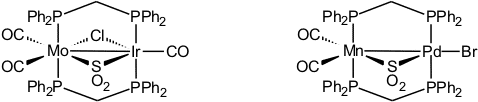Zweikernkomplexe
Bimetallic Complexes
Complexes in which two metal atoms of different electron count are held in close proximity seem to offer fascinating opportunities to achieve novel types of reactions. In order to preserve the integrity of the binuclear complex it is advisable to tie the two metal atoms together with bridging ligands. Phosphino-substituted cyclopentadienes have proven to be particularly reliable braces to connect a titanium or zirconium atom to a late transition metal. A few examples of compounds which offer new opportunities are listed below:
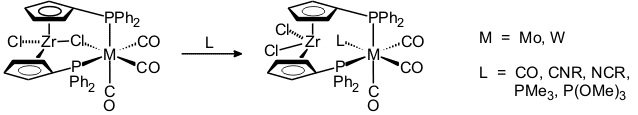
Chloride-bridged binuclear complexes rapidly add small ligands such as CO, nitriles, isonitriles, PMe3, or P(OMe)3 [41, 51, 80].
The coupling of a zirconium-bound methyl group with two tungsten-bound nitrile ligands was observed in the following reaction [80]:
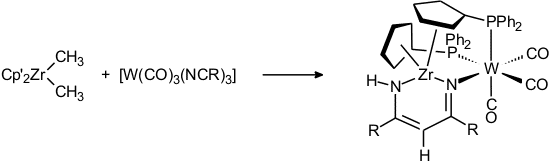
Further publications describe the synthesis and insertion reactions of cyclohexyne-zirconium-molybdenum complexes [81] and metal-metal bonded zirconium-rhenium complexes [82].
Bis(diphenylphosphino)methane is the ligand of choice for connecting two soft transition metal centers. The systems we have investigated involve the metal combinations Mo/Rh, Mo/Ir, Mo/Pd, W/Pd, Mn/Pd, Fe/Rh, Rh/Pt, Ir/Pd, and Ir/Pt [42, 50]. One of our goals was to define the properties of sulfur dioxide (see sulfur chemistry) as a bridging ligand.