Thioaldehyde
Thioaldehydes
Transition metal complexes of thioaldehydes have been known for quite a while. We have some time ago found a new access to this class of compounds consisting of a beta-hydride abstraction [56].
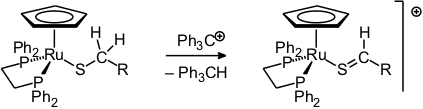
With strongly electron-donating phosphine ligands at the ruthenium atom, rapid eta1/eta2 coordination equilibria were found [67], whereas the similar rhenium complexes exist predominantly as eta2 isomers [70, 86, 96]. The rhenium complexes are particularly suited to stabilize aliphatic thioaldehydes including those containing various functional groups in the side chain [91, 93, 96, 119].
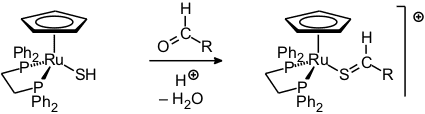
A second method of synthesis which is also applicable to complexes of unsaturated thioaldehydes as well as thioketones is based on an acid-catalyzed condensation reaction [86, 114, 117, 123].
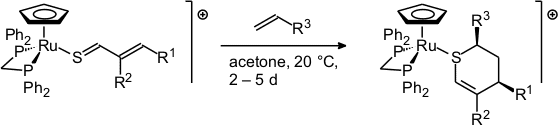
Although these "stabilized" thioaldehydes are less reactive than the uncoordinated ones, they still undergo nucleophilic additions and [2+4]-cycloadditions [67, 86, 114, 123].

![Structure of the cation of [CpRu(dppe)(eta1-S=CHC6H4OMe)]PF6 [67]](/fileadmin/_processed_/5/5/csm_s06_2c53aa1b7c.jpg)
![Structure of the cation of [CpRe(NO)(PPh3)2(eta2-S=CHPh)]PF6 [70]](/fileadmin/_processed_/8/3/csm_s07_cfa33451f6.jpg)