Schwefeloxide
Sulfur Oxides
Early work on the coordination chemistry of the sulfur oxides began with carbonyl-SO2 complexes of chromium, molybdenum, and tungsten [13, 18, 19, 22, 30] (see Metal Carbonyls). Our main interest was the interrelationship between structure, spectroscopy, and the various bonding modes of SO2.
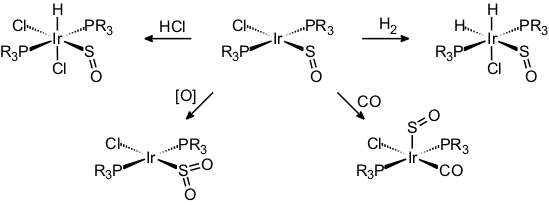
Our work on the coordination chemistry of sulfur monoxide began with the isolation of the square-planar compounds [MCl(PR3)2(SO)] (M = Rh, Ir) which had been synthesized using thiirane-1-oxide as an SO-transfer reagent. These were the first mononuclear complexes of sulfur monoxide to be rigorously characterized, including a crystal structure determination [24].
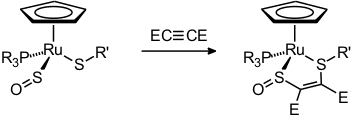
Later came a few halfsandwich-type complexes of iron and ruthenium [32, 43, 46, 58]. Typical reactions of these species are the oxidation of the coordinated SO to SO2 [31], coordinative and oxidative addition at iridium [25, 36], the liberation and trapping of SO [35], and one example of a [3+2]-cycloaddition [58].

![Molecular Structure of [Cr(CO)5(SO2)] [18]](/fileadmin/_processed_/9/6/csm_s01_d03dd3554e.jpg)
![Molecular Structure of [Mo(CO)2(PMe3)3(eta2-SO2)] [30]](/fileadmin/_processed_/3/6/csm_s02_7cd12cee68.jpg)
![Structure of the cation of [Cp*Ru(PMe3)2(eta2-H2C=SO2)]PF6 [62]](/fileadmin/_processed_/d/3/csm_s03_a12540ad82.jpg)
![Structure of the cation of [Cp*Ru(PMe3)2(h2-O=SO2)]PF6 [73]](/fileadmin/_processed_/e/b/csm_s04_46ffa81107.jpg)
![Molecular Structure of [IrCl(Pi-Pr3)2(SO)] [24]](/fileadmin/_processed_/b/f/csm_s05_7662349c8e.jpg)