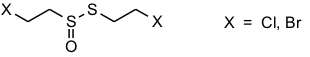Tin Chemistry
Tin Chemistry
Organotin halides form addition compounds with a wide range of Lewis acids. We have been particularly interested in the ligand properties of thiirane-1-oxide which we had used as a source of sulfur monoxide (see Sulfur Chemistry). Indeed, a large number of addition compounds [SnX4(C2H4SO)2], [RSnX3(C2H4SO)2], [R2SnX2(C2H4SO)2], [R2SnX2(C2H4SO)], and [R3SnX(C2H4SO)] (X = Cl, Br; R = Me, t-Bu, Ph, substituted aryl) have been synthesized and characterized [40, 48, 66]. The thermodynamics of the equilibrium
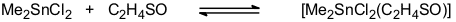
have been evaluated by variable-temperature NMR. The results indicate that thiirane-1-oxide is a slightly weaker donor than DMSO [66]. Thermal decomposition of some of these adducts provides an easy access to chloro- or bromo-substituted esters of thiosulfinic acids.