Just Published in Chemistry - A European Journal
04/04/2017Self-Assembly of 9,10-Bis(phenylethynyl) Anthracene (BPEA) Derivatives: Influence of π–π and Hydrogen-Bonding Interactions on Aggregate Morphology and Self-Assembly Mechanism
Authors: Michael Lübtow, Ingo Helmers, Dr. Vladimir Stepanenko, Dr. Rodrigo Q. Albuquerque, Prof. Dr. Todd B. Marder, Prof. Dr. Gustavo Fernández
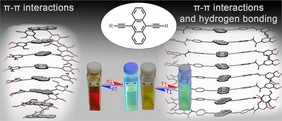
Abstract: 9,10-Bis(phenylethynyl)anthracenes (BPEAs) are an important class of dyes in various applications including chemiluminescence emitters, materials for photon upconversion, and for optoelectronic devices. Some of these applications require control over the packing modes of the molecules within the active layer, which can be effected by bottom-up self-assembly. Studies aimed at controlling the molecular organization of BPEAs have primarily focused on bulk or liquid-crystal materials, whereas in-depth investigations of BPEA-based assemblies in solution remain elusive. In this article, the self-assembly of two new BPEA derivatives with hydrophobic side chains is reported, one of them featuring amide functional groups and the other one lacking them. Comparison of the self-assembly behavior of both systems in solution using spectroscopic (UV/Vis, fluorescence, and NMR), microscopic (AFM), and theoretical (PM6) studies reveals the crucial role of the amide groups in controlling the self-assembly. For both systems, the formation of H-type face-to-face π-stacks is proposed, whereas the interplay of π-stacking and H-bonding is responsible for driving the formation of 1D stacks and increasing the binding constant by two to three orders of magnitude. Our findings show that H-bonding is a prerequisite to create ordered BPEA assemblies in solution.
Link: http://onlinelibrary.wiley.com/doi/10.1002/chem.201605989/abstract