Just Published in Angewandte Chemie, International Edition
12/19/20181,2,3‐Diazaborinine: A BN Analogue of Pyridine Obtained by Ring Expansion of a Borole with an Organic Azide
Authors: Felix Lindl, Shujuan Lin, Ivo Krummenacher, Carsten Lenczyk, Andreas Stoy, Marcel Müller, Zhenyang Lin, Holger Braunschweig*
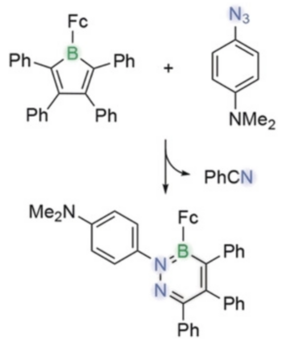
Abstract: A new pathway for the ring expansion reaction of antiaromatic boroles with organic azides is reported. While the reaction usually leads to 1,2‐azaborinines, it was diverted to the formation of a 1,2,3‐diazaborinine by changing the electronic characteristics of the reagents. The isolable azo‐azaborinine intermediate initially formed from the reaction of 1‐(2,3,4,5‐tetraphenylborolyl)ferrocene with 4‐azido‐N,N‐dimethylaniline gradually decomposed to a 1,2,3‐diazaborinine and benzonitrile. Both the spectroscopic properties and the reactivity of the heteroaromatic compound show analogies to pyridine, to which it is isoelectronic. Density functional theory (DFT) calculations provided insight into the mechanism of this unusual transformation.
Link: https://onlinelibrary.wiley.com/doi/full/10.1002/anie.201811601