Exciton Coupling of Merocyanine Dyes from H- to J‑type in the Solid NANOletters. 2017 , A key issue for the application of π -conjugated organic
Solvent-Templated Folding of Perylene Bisimide Macro- cycles into Coiled Double-String Ropes with Solvent-Sensitive Optical Signatures J. Am. Chem. Soc. 2017 , DOI:10.1021/jacs.6b11973
A Columnar Liquid-Crystal Phase Formed by Hydrogen-Bonded Perylene Bisimide J-Aggregate 2017 ,56 , Early ViewDOI:10.1002/anie.201612047 p - p interactions into J-aggregates which in turn organize into liquid-crystalline columnar domains. The dye molecules organize with the cores parallel to the columnar axis forming an unprecedented triple stranded helical structure with transition dipoles m ag pointing along the columnar axis.
Direct Observation of Excimer-Mediated Intra- molecular Electron Transfer in a Cofacially Stacked Perylene Bisimide Pair J.Am.Chem.Soc. 2016 Early ViewDOI:10.1021/jacs.6b04591 - mediated intramolecular electron transfer in cofacially - stacked PBIs tethered by two phenylene - butadiynylene loops. The coexistence of the PBI radical anion and cation bands in the transient absorption spectra reveals an electron transfer (ET) between two identical PBI moieties, i.e. symmetry - breaking ET similar as given in the special BChl pair in photosynthetic reaction centers.
>A Supramolecular Ruthe- nium Macrocycle with High Catalytic Activity for Water Oxidation that Mechanis- tically Mimics Photosystem II Nat.Chem . 2016 DOI:10.1038/NCHEM.2503
>An Electron-Poor C64 Nano- graphene by Palladium-Catalyzed Cascade C –C Bond Formation: One Pot Synthesis and Single-Crystal Structure Analysis Angew.Chem. Int. Ed . 2016 ,55 , Early ViewDOI:10.1002/anie.201601433 64 nanographene was revealed by single-crystal X-ray analysis.
>Perylene Bisimide Dye Assemblies as Archetype Functional Supramolecular Materials Chem.Rev. 2016 ,116 ,962–1052DOI:10.1021/acs.chemrev.5b00188
>Direct Observation of Ultrafast Coherent Exciton Dynamics in Helical pi-Stacks of Self-Assembled Perylene Bisimide Nat.Commun. 2015 ,6 ,8646DOI:10.1038/ncomms9646
>Shape-Controlled Synthesis and Self-Sorting of Covalent Organic Cage Compounds Angew. Chem. Int. Ed. 2015 , 54 , 10356-10360DOI: 10.1002/anie.201502983
The directional bonding approach is a powerful tool to rationally control both shape and stoichiometry of three-dimensional objects built from rigid building blocks under dynamic covalent conditions. Co-condensation of catechol-functionalized tribenzotriquinacene derivatives which have 90° angles between the reactive sites and diboronic acids with bite angles of 60°, 120°, and 180°, led to the efficient formation of, respectively, bipyramidal, tetrahedral, or cubic covalent organic cage compounds in a predictable manner. Investigations on the self-sorting of ternary mixtures containing two competitive boronic acids revealed either narcissistic or social self-sorting depending on the stability of the segregated cages relative to feasible three-component assemblies.
>Head-to-Tail Zig-Zag Packing of Dipolar Merocyanine Dyes Affords High-Performance Organic Thin-Film Transistors Angew. Chem. 2015 ,127 ,10658;2015 ,54 DOI:10.1002/anie.201504190
2 V- 1 s- 1 . Because of the optical properties and the suitable orientation of the transition dipoles parallel to the substrate, theses molecules might also be promising for organic photovoltaic applications.
>A Perylene Bisimide Cyclo- phane as “Turn-on” and “Turn-off” Fluorescence Probe Angew.Chem .2015 ,127 ,10303–10306; Angew.Chem.Int.Ed .2015 ,54 ,10165
DOI:10.1002/anie.201503542
A perylene bisimide cyclophane was synthesized that proved to be capable to intercalate large planar aromatic hydrocarbons. The fluorescence properties of the corresponding host-guest complexes are strongly dependent on the electronic structure of the guest molecules. Therefore, the reported system serves as “turn-on” and “turn-off” fluorescence probe distinguishing electron-poor from electron-rich aromatic hydrocarbons.
>Bright Fluorescence and Host-Guest Sensing with a Nanoscale M4 L6 Tetrahedron Accessed by Self-Assembly of Zinc-Imine Chelate Vertices and Perylene Bisimide Edges P. Frischman, V. Kunz, F. WürthnerAngew.Chem .2015 ,127 ,7393–7397; Angew.Chem.Int.Ed .2015 ,54 ,7285
DOI:10.1002/anie.201501670
A highly luminescent Zn4 L6 tetrahedron is reported with the rare combination of structural integrity at concentrations <10-7 mol L-1 and an excep- tionally high fluorescence quantum yield of Φem = 0.67. Encapsulation of multiple perylene or coronene guest molecules is accompanied by strong luminescence quenching.
>Supramolecular Block Copolymers by Kinetically Controlled Co-Self-Assembly of Planar and Core-Twisted Perylene Bisimides D.Görl,X.Zhang,V.Stepanenko,F. WürthnerNat.Commun .2015 ,6 ,7009DOI:10.1038/ncomms8009 m BB)n . The highly defined ultralong nano- wire structure of these supramolecular copolymers entirely differs from those formed upon self-assembly of the individual counterparts and constitutes a kinetic self- assembly product that transforms into thermody- namically more stable self- sorted homopolymers upon heating.
>Mechanism of Self- Assembly Process and Seeded Supramolecular Polymerization of Perylene Bisimide Organogelator S.Ogi,V.Stepanenko,K.Sugiyasu, M.Takeuchi, F.WürthnerJ.Am.Chem.Soc .2015 ,137 ,3300–3307DOI:10.1021/ja511952c
> An Ambient Stable Core- Substituted Perylene Bisimide Dianion: Isolation and single Crystal Structure Analysis S. Seifert, D.Schmidt, F. WürthnerChem.Sci. 2015 ,6 ,1663–1667DOI:101039/c4sc03671a
>Near-IR Phosphorescent Ruthenium(II) and Iridium(III) Perylene Bisimide Metal Complexes Angew. Chem. 2015 , 127 , 1590–1593; Angew. Chem. Int. Ed. 2015 , 54 , 1570–1573DOI:10.1002/anie.201410437 PBI-metal complexes in which the spin- orbit coupling is strong enough to facilitate not only the Sn à Tn intersystem crossing of the PBI dye, but also the radiative T1 à S0 transition, i.e. phosphorescence.
>Single-crystal field-effect transistors of new Cl2 -NDI polymorph processed by sublimation in air Nat. Commun. 2015 , 6, 1-9 Doi:10.1038/ncomms6954
Nature Communications here reports about a better electricity conductor than all other comparable materials. It holds the record among small mole- cules in terms of the charge-carrier mobility of electrons in air. It enables a new manu- facturing technique thus opening up a new field of work.
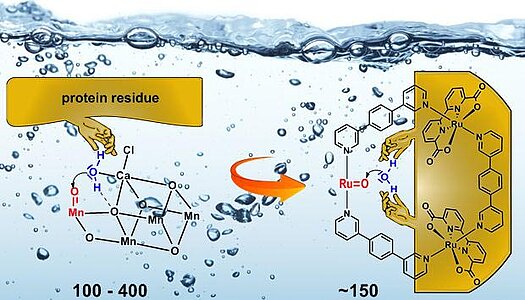
>Embedding of Ruthenium (II) Water Oxidation Catalyst into Nanofibers via Self-Assembly Chem. Commun. 2015 , 51 290 – DOI: 10.1039/c44c08314h
A novel ruthenium(II) water oxidation catalyst equipped with axial perylene bisimide ligands that self-assembled into nanofibers was synthesized. This fiber-embedded catalyst exhibited improved performance compared with a monomeric reference for the oxidative water splitting reaction.
>Dynamic covalent assembly of tribenzotriquinacenes into molecular cubes Comm. Comm , 2014 , Advance Article DOI: 10.1039/C4CC01794C
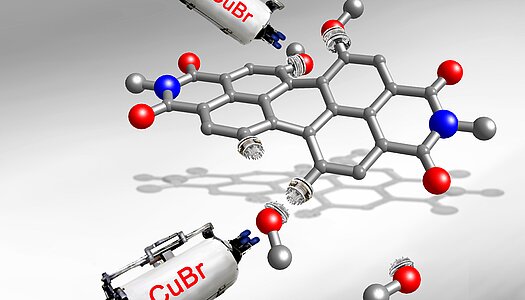
>Strategies for the Synthesis of Functional Naphthalene Diimides Angew.Chem. 2014 , 126, 7558 – 7578DOI: 10.1002/anie.20130974
In this Review we have highlighted the enormous increase in the number of chemical structures derived from the parent naphthalene diimide, which has only been explored over a few years, fueled by great interest in organic n-channel semiconductors.
>Synthesis and Properties of a New Class of Fully Conju- Angew.Chem. 2014 ,126 ,6273–6276; Angew.Chem. Int. Ed . 2014 , 53 ,6259–6162DOI:10.1002/anie.201403227
>Multidimensional spectroscopy of photoreactivity T. Brixner et al.PNAS , 2014 , early edition, 1-6
doi/10.1073/pnas.1323792111
>Stepwise Decrease of Fluorescence versus Sequential Photobleaching in a Single Multi-chromophoric System 2014 , 8 , 1708-1717
DOI: 10.1021/nn4060946all neutral subunits within a trimer rather than exclusively from the subunit with the lowest site energy.
>An Organogelator Design without Solubilizing Side Chains by Backbone Contortion of Perylene Bisimide Pigment Z. Xie et al.Mater. Horiz. , 2014 ,1 , 355-359DOI:10.1039/c3mh00159h
>High-Performance Organic Thin-Film Transistors of J-Stacked Squaraine Dyes J. Am. Chem. Soc. , 2014 , 136 , 2351-2362
We have synthesized a series of dipolar squaraine dyes that contain dicyanovinyl groups as
>Hierarchical Growth of Fluorescent Dye Aggregates in Water by Fusion of Segmented Nanostructures Angew.Chem. 2014 , 126 , 1294–1298; Angew. Chem. Int. Ed . 2014 , 53 , 1270–1274
DOI:10.1002/anie.201308963
We elucidate the supramolecular growth process for an outstanding class of functional dyes, perylene bisimides (PBIs), by TEM, cry-SEM, and AFM. Our studies reveal a sequential growth of amphiphilic PBI dyes from nanorods into nanoribbons in water by fusion and fission processes.
>Self-Assembly and (Hydro)gelation Triggered by Cooperative π–π and Unconventional C Angew. Chem. 2014 , 126, 716–722; Angew. Chem. Int. Ed . 2014 , 53 , 700–705
A new oligophenyleneethynylene (OPE)-based amphiphilic Pt(II) complex has been synthesized that forms supramolecular polymeric structures in aqueous and polar media driven by multiple unconventional C-H···X (X=O, Cl) hydrogen bonding interactions, assisted by π– π interactions. This interplay between different weak noncovalent forces leads to the cooperative formation of self-assembled structures of high aspect ratio and gels in which the molecular arrangement is maintained in the crystalline state
>Structure–property relation- A. J. Jiménez et al.Chem. Sci. 2014 , 5 , 608–619
DOI:10.1039/c3sc52344f
To elucidate the impact of widely employed solubilizing phenoxy substituents on the structural and functional properties of perylene bisimide (PBI) dyes a series of 1,7-diphenoxy-substituted PBIs was prepared from 1,7-dibromo PBI which exhibit hydrogen, methyl, isopropyl or phenyl substituents at one or both
ortho positons of the phenoxy substituents.
>Air-Stable n-Channel Organic Single Crystal Field-Effect Transistors Based on Microribbons
T. He et al.Adv.Mater. 2013 , 25 , 6951–6955DOI: 10.1002/adma.201303392
Ribbon-shaped single crystal transistors based on naphthalene diimide Cl2 -NDI exhibit excellent n-channel performance with the mobility as high as 8.6 cm2 V−1 s−1 in air. The combination of ambient stability and high mobility n-channel transport closes the gap between p- and n-channel SCFETs and opens the door for the manufacture of high performance complementary organic circuits.
>Chlorophyll J-Aggregates: From Bioinspired Dye Stacks to Nanotubes, Liquid Crystals, and Biosupra- S. Sengupta et. al.Acc. Chem. Res. 2013 , 46 , 2498–2512DOI:10.1021/ar400017u
> 4 L6 Tetrahedral Host Self-J. Am. Chem . Soc. 2013 ,135 ,15656–15661DOI: 10.1021/ja/4083039
>Backbone-Directed Pery- Angew. Chem . 2013 , 125 , Angew .Chem. Int. Ed . 2013 , 52 ,10463–10467DOI:10.1002/ange.201305894
>Quadruple π Stacks of Two Perylene Bisimide Tweezers: A Bimolecular Complex with Kinetic Stab ility Angew. Chem . 2013 , 125 , 7630–7634; Angew. Chem. Int. Ed. 2013 , 52 , 7482–7486DOI: 10.1002/anie.201302479
>Evidence for Kinetic Nucle- 2013 ,19 ,4176–4183DOI: 10.1002/chem.201204146
>Enhanced photocurrent generation by folding-driven H-aggregate formation Chem. Sci. 2013 , 4 , 2071–2075DOI: 10.1039/C3SC50263E A novel bis(merocyanine) dye ha s been synthesized whose folding and aggregation behavior afforded extended cofacially pi -stacked H-aggregates that lead to enhanced generation of photocurrent in BHJ solar cells.
>Cooperative Supra-molecular Polymerization Driven by Metallophilic Pd···Pd Interactions 2013 , 135 , 2148−2151
A new oligophenylene-ethynylene (OPE)-based Pd(II) pyridyl complex has been synthesized, and itsself-assembly has been investigated in solution, in the bulk state, and on surfaces. Detailed analysis of concentrationand temperature-dependent UV−vis studies in methylcyclohexanesupported by DFT calculations demonstrate for the first time that cooperative supramolecular polymerization processes can be driven by metallophilic interactions.
>Powering the future of molecular artificial photosynthesis with light-harvesting metallo- P. D. Frischmann et al.Chem. Soc. Rev. 2013 , 42 , 1847–1870DOI: 10.1039/C2CS35223K
>Bay-substituted perylene bisimide dye with an undistorted planar scaffold Chem. Commun. 2012 , 48 , 12050–12052DOI:10.1039/c2cc36719j
> Perylene Bisimide Dimer Aggregates: Chem. Eur. J. 2012 , 18 , 13665–13677DOI:10.1002/chem.201201661
>Biosupramolecular Nanowires from Chlorophyll Dyes with Exceptional Charge-Transport Properties S. Sengupta et al.Angew. Chem. 2012 , 124 , 6484–6488; Angew. Chem . Int. Ed. 6378–6382
DOI:10.1002/anie.201201961
Conductive tubes: Self-assembled nanotubes of a bacteriochlorophyll derivative are reminiscent of natural chlorosomal light-harvesting assemblies. After deposition on a substrate that consists of a non-conductive silicon oxide surface (see picture, brown) and contacting the chlorin nanowires to a conductive polymer (yellow), they show exceptional charge-transport properties.
>Molecular Assemblies of Perylene Bisimide Dyes in Water D. Görl, X. Zhang, F. WürthnerAngew. Chem. 2012 , 124 , 6434–6455; Angew. Chem. Int. Ed . 2012 , 51 , 6328–6348
DOI:10.1002/ange.201108690
Fascinating functional nanostrucures are formed in water by amphiphilic perylene bisimide dyes through strong hydrophobic interactions. This Review describes the current developments in the self-assembly of perylene bisimides in water to form π–π-stacked molecular aggregates.
>Halogen-Arene Interactions Assist in Self-Assembly of Dyes Angew. Chem. 2012 , 124 , 5713–5717; Angew. Chem. Int. Ed. 2012 , 51 , 5615–5619DOI:10.1002/anie.201200897
The "dye" is cast: An isodesmic self-assembly process of near-infared-absorbing, acceptor-substituted squaraine dyes, assisted by halogen–halogen and halogen–arene interactions, is observed in toluene. The aggregation process was monitored by UV/Vis/NIR absorption and NMR experiments, thus providing insight into the structural features (see figure; O red, I violet, N blue) as well as the binding strengths of the formed aggregates.
>Chiral J-Aggregates of Atropo-Enentiomeric Perylene Bisimides and Their Self-Sorting Behavior Chem. Eur. J . 2012 , 18 , 7060–7070DOI:10.1002/chem.201200089
Homochiral and heterochiral J-aggregates were created by nucleation-elongation assembly of atropo-enentiomerically pure and racemic perylene bisimides, which showed quite different morphological and optical properties. One dimensional helical nanowires with high photoluminescence efficiency were observed from the homochiral J-aggregates (see figure).
> Metallosupramolecular amphiphilic pi-systems G. Fernández 2012 , 3 , 1395-1398DOI:10.1039/c2sc01101h
Metallosupramolecular amphiphiles are emerging as a new class of adaptive materials with the ability to self-assemble into a wide variety of supramolecular structures through simultaneous pi-metallophilic and metal-ligand interactions.
> Foldamer with a spiral perylene bisimide staircase V. Dehm, M. Büchner, J. Seibt, V. Engel, F. WürthnerChem.Sic. 2011 , 2 , 2094–2100
DOI: 10.1039/c1sc00435b
A molecular staircase was constructed by a new foldamer concept based on intramolecular π–π-interactions of perylene bisimide dyes.
> Self-Sorting Phenomena in Complex Supramolecular Systems M. M. Safont-Sempere, G. Fernández, F. WürthnerChem. Rev. 2011 , 111 , 5784–5814
DOI: 10.1021/cr100357h
In this review, we discuss the external variables and intrinsic factors (molecular codes) that influence the recognition or discrimination of supramolecularly interacting chemical species in solution. The comprehension of this “molecular programming” in artificial systems will define the variables that control self-sorting processes, and may ultimately contribute to a better understanding of the self-assembly pathways in natural systems.
Shape-Controlled Synthesis and Self-Sorting of Covalent Organic Cage Compounds Angew. Chem. Int. Ed. 2015 , 54 , 10356-10360DOI: 10.1002/anie.201502983
The directional bonding approach is a powerful tool to rationally control both shape and stoichiometry of three-dimensional objects built from rigid building blocks under dynamic covalent conditions. Co-condensation of catechol-functionalized tribenzotriquinacene derivatives which have 90° angles between the reactive sites and diboronic acids with bite angles of 60°, 120°, and 180°, led to the efficient formation of, respectively, bipyramidal, tetrahedral, or cubic covalent organic cage compounds in a predictable manner. Investigations on the self-sorting of ternary mixtures containing two competitive boronic acids revealed either narcissistic or social self-sorting depending on the stability of the segregated cages relative to feasible three-component assemblies.